Abstract
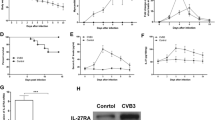
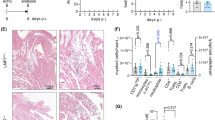
Coxsackieviruses of group B (CVB) are well-known causes of acute and chronic myocarditis. Chronic myocarditis can evolve into dilated cardiomyopathy (DCM) characterized by fibrosis and cardiac remodeling. Interleukin-1β (IL-1β) plays a decisive role in the induction of the inflammatory response as a consequence of viral replication. In this study, we analyzed the effects of IL-1β neutralization on the transition of acute to chronic myocarditis in a mouse model of CVB3 myocarditis. Mice were treated with an anti-murine IL-1β antibody as a surrogate for Canakinumab at different time points post CVB3 infection. Treatment was performed in the early phase (day 1–14 pi, day 3–14 pi) or at a later stage of myocarditis (day 14–28 pi). Subsequently, the hearts were examined histologically, immunohistochemically and by molecular biology. A significant reduction of viral replication, cardiac damage and inflammation was found after administration of the antibody in the early phase and in the later phase of infection. Furthermore, less collagen I deposition and a considerable reduction of fibrosis were found in antibody-treated mice. Using microarray analysis, a significant upregulation of various extracellular matrix and fibrosis-associated molecules was found in CVB3-infected mice, including TGF-β, TIMP-1 and MMP12, as well as diverse matricellular proteins, whereas, these molecules were significantly downregulated in all IL-1β antibody-treated infected mice. Neutralization of IL-1β at different stages of enteroviral infection prevents the development of chronic viral myocarditis by reducing inflammation, interstitial fibrosis and adverse cardiac remodeling. These findings are relevant for the treatment of patients with acute and chronic myocarditis.










Similar content being viewed by others
References
Al-Kofahi M, Omura S, Tsunoda I, Sato F, Becker F, Gavins FNE, Woolard MD, Pattillo C, Zawieja D, Muthuchamy M, Gashev A, Shihab I, Ghoweba M, Von der Weid PY, Wang Y, Alexander JS (2018) IL-1beta reduces cardiac lymphatic muscle contraction via COX-2 and PGE2 induction: Potential role in myocarditis. Biomed Pharmacother 107:1591–1600. https://doi.org/10.1016/j.biopha.2018.08.004
Althof N, Goetzke CC, Kespohl M, Voss K, Heuser A, Pinkert S, Kaya Z, Klingel K, Beling A (2018) The immunoproteasome-specific inhibitor ONX 0914 reverses susceptibility to acute viral myocarditis. EMBO Mol Med 10:200–218. https://doi.org/10.15252/emmm.201708089
Baldeviano GC, Barin JG, Talor MV, Srinivasan S, Bedja D, Zheng D, Gabrielson K, Iwakura Y, Rose NR, Cihakova D (2010) Interleukin-17A is dispensable for myocarditis but essential for the progression to dilated cardiomyopathy. Circ Res 106:1646–1655. https://doi.org/10.1161/circresaha.109.213157
Caforio AL, Pankuweit S, Arbustini E, Basso C, Gimeno-Blanes J, Felix SB, Fu M, Helio T, Heymans S, Jahns R, Klingel K, Linhart A, Maisch B, McKenna W, Mogensen J, Pinto YM, Ristic A, Schultheiss HP, Seggewiss H, Tavazzi L, Thiene G, Yilmaz A, Charron P, Elliott PM (2013) Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 34:2636–2648. https://doi.org/10.1093/eurheartj/eht210
Cavalli G, Foppoli M, Cabrini L, Dinarello CA, Tresoldi M (2017) Dagna L interleukin-1 receptor blockade rescues myocarditis-associated end-stage heart failure. Front Immunol 8:131. https://doi.org/10.3389/fimmu.2017.00131
Cavalli G, Pappalardo F, Mangieri A, Mangieri A, Dinarello CA, Dinarello CA, Dagna L, Dagna L, Tresoldi M (2016) Treating life-threatening myocarditis by blocking interleukin-1. Crit Care Med 44:e751–e754. https://doi.org/10.1097/ccm.0000000000001654
Cheung C, Luo H, Yanagawa B, Leong HS, Samarasekera D, Lai JC, Suarez A, Zhang J, McManus BM (2006) Matrix metalloproteinases and tissue inhibitors of metalloproteinases in coxsackievirus-induced myocarditis. Cardiovasc Pathol 15:63–74. https://doi.org/10.1016/j.carpath.2005.11.008
Diaz JA, Booth AJ, Lu G, Wood SC, Pinsky DJ, Bishop DK (2009) Critical role for IL-6 in hypertrophy and fibrosis in chronic cardiac allograft rejection. Am J Transplant 9:1773–1783. https://doi.org/10.1111/j.1600-6143.2009.02706.x
Dinarello CA (2009) Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol 27:519–550. https://doi.org/10.1146/annurev.immunol.021908.132612
Dinarello CA (2011) Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood 117:3720–3732. https://doi.org/10.1182/blood-2010-07-273417
EMEA (2009) CHMP assessment report for Ilaris. London. https://www.ema.europa.eu/documents/assessment-report/ilaris-epar-public-assessment-report_en.pdf. Accessed 23 July 2009
Emmi G, Urban ML, Imazio M, Gattorno M, Maestroni S, Lopalco G, Cantarini L, Prisco D, Brucato A (2018) Use of Interleukin-1 blockers in pericardial and cardiovascular diseases. Curr Cardiol Rep 20:61. https://doi.org/10.1007/s11886-018-1007-6
Frangogiannis NG (2012) Matricellular proteins in cardiac adaptation and disease. Physiol Rev 92:635–688. https://doi.org/10.1152/physrev.00008.2011
Frustaci A, Russo MA, Chimenti C (2009) Randomized study on the efficacy of immunosuppressive therapy in patients with virus-negative inflammatory cardiomyopathy: the TIMIC study. Eur Heart J 30:1995–2002. https://doi.org/10.1093/eurheartj/ehp249
Huber M, Watson KA, Selinka HC, Carthy CM, Klingel K, McManus BM, Kandolf R (1999) Cleavage of RasGAP and phosphorylation of mitogen-activated protein kinase in the course of coxsackievirus B3 replication. J Virol 73:3587–3594
Klingel K, Fabritius C, Sauter M, Goldner K, Stauch D, Kandolf R, Ettischer N, Gahlen S, Schonberger T, Ebner S, Makrigiannis AP, Belanger S, Diefenbach A, Polic B, Pratschke J, Kotsch K (2014) The activating receptor NKG2D of natural killer cells promotes resistance against enterovirus-mediated inflammatory cardiomyopathy. J Pathol 234:164–177. https://doi.org/10.1002/path.4369
Klingel K, Hohenadl C, Canu A, Albrecht M, Seemann M, Mall G, Kandolf R (1992) Ongoing enterovirus-induced myocarditis is associated with persistent heart muscle infection: quantitative analysis of virus replication, tissue damage, and inflammation. Proc Natl Acad Sci U S A 89:314–318. https://doi.org/10.1073/pnas.89.1.314
Krueger GR, Ablashi DV (2003) Human herpesvirus-6: a short review of its biological behavior. Intervirology 46:257–269. https://doi.org/10.1159/000073205
Kuhl U, Pauschinger M, Schwimmbeck PL, Seeberg B, Lober C, Noutsias M, Poller W, Schultheiss HP (2003) Interferon-beta treatment eliminates cardiotropic viruses and improves left ventricular function in patients with myocardial persistence of viral genomes and left ventricular dysfunction. Circulation 107:2793–2798. https://doi.org/10.1161/01.cir.0000072766.67150.51
Kuhl U, Pauschinger M, Seeberg B, Lassner D, Noutsias M, Poller W, Schultheiss HP (2005) Viral persistence in the myocardium is associated with progressive cardiac dysfunction. Circulation 112:1965–1970. https://doi.org/10.1161/circulationaha.105.548156
Lane JR, Neumann DA, Lafond-Walker A, Herskowitz A, Rose NR (1993) Role of IL-1 and tumor necrosis factor in coxsackie virus-induced autoimmune myocarditis. J Immunol 151:1682–1690
Lim BK, Choe SC, Shin JO, Ho SH, Kim JM, Yu SS, Kim S, Jeon ES (2002) Local expression of interleukin-1 receptor antagonist by plasmid DNA improves mortality and decreases myocardial inflammation in experimental coxsackieviral myocarditis. Circulation 105:1278–1281. https://doi.org/10.1161/01.CIR.0000012492.06971.88
Luo H, Yanagawa B, Zhang J, Luo Z, Zhang M, Esfandiarei M, Carthy C, Wilson JE, Yang D, McManus BM (2002) Coxsackievirus B3 replication is reduced by inhibition of the extracellular signal-regulated kinase (ERK) signaling pathway. J Virol 76:3365–3373. https://doi.org/10.1128/JVI.76.7.3365-3373.2002
Ma F, Li Y, Jia L, Han Y, Cheng J, Li H, Qi Y, Du J (2012) Macrophage-stimulated cardiac fibroblast production of IL-6 is essential for TGF β/Smad activation and cardiac fibrosis induced by angiotensin II. PLoS One 7:e35144. https://doi.org/10.1371/journal.pone.0035144
Marchant DJ, McManus BM (2010) Regulating viral myocarditis: allografted regulatory T cells decrease immune infiltration and viral load. Circulation 121:2609. https://doi.org/10.1161/CIRCULATIONAHA.110.960054
Maron BJ, Towbin JA, Thiene G, Antzelevitch C, Corrado D, Arnett D, Moss AJ, Seidman CE, Young JB (2006) Contemporary definitions and classification of the cardiomyopathies. Circulation 113:1807. https://doi.org/10.1161/CIRCULATIONAHA.106.174287
Nakano A, Matsumori A, Kawamoto S, Tahara H, Yamato E, Sasayama S, Miyazaki JI (2001) Cytokine gene therapy for myocarditis by in vivo electroporation. Hum Gene Ther 12:1289–1297. https://doi.org/10.1089/104303401750270940
Neumann DA, Lane JR, Allen GS, Herskowitz A, Rose NR (1993) Viral myocarditis leading to cardiomyopathy: do cytokines contribute to pathogenesis? Clin Immunol Immunopathol 68:181–190. https://doi.org/10.1006/clin.1993.1116
Osborn O, Brownell SE, Sanchez-Alavez M, Salomon D, Gram H, Bartfai T (2008) Treatment with an interleukin 1 beta antibody improves glycemic control in diet-induced obesity. Cytokine 44:141–148. https://doi.org/10.1016/j.cyto.2008.07.004
Pappritz K, Savvatis K, Miteva K, Kerim B, Dong F, Fechner H, Muller I, Brandt C, Lopez B, Gonzalez A, Ravassa S, Klingel K, Diez J, Reinke P, Volk HD, Van Linthout S, Tschope C (2018) Immunomodulation by adoptive regulatory T-cell transfer improves Coxsackievirus B3-induced myocarditis. Faseb J. https://doi.org/10.1096/fj.201701408r
Parisi F, Paglionico A, Varriano V, Ferraccioli G, Gremese E (2017) Refractory adult-onset still disease complicated by macrophage activation syndrome and acute myocarditis: a case report treated with high doses (8 mg/kg/d) of anakinra. Med (Baltim) 24:e6656. https://doi.org/10.1097/md.0000000000006656
Pollack A, Kontorovich AR, Fuster V, Dec GW (2015) Viral myocarditis–diagnosis, treatment options, and current controversies. Nat Rev Cardiol 12:670–680. https://doi.org/10.1038/nrcardio.2015.108
Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ (2017) Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 377:1119–1131. https://doi.org/10.1056/NEJMoa1707914
Rondeau J-M, Ramage P, Zurini M, Gram H (2015) The molecular mode of action and species specificity of canakinumab, a human monoclonal antibody neutralizing IL-1β. MAbs 7:1151–1160. https://doi.org/10.1080/19420862.2015.1081323
Shimojo N, Hashizume R, Kanayama K, Hara M, Suzuki Y, Nishioka T, Hiroe M, Yoshida T, Imanaka-Yoshida K (2015) Tenascin-C may accelerate cardiac fibrosis by activating macrophages via the integrin alphaVbeta3/nuclear factor-kappaB/interleukin-6 axis. Hypertension 66:757–766. https://doi.org/10.1161/hypertensionaha.115.06004
Sternlicht MD, Werb Z (2001) How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol 17:463–516. https://doi.org/10.1146/annurev.cellbio.17.1.463
Szalay G, Sauter M, Haberland M, Zuegel U, Steinmeyer A, Kandolf R, Klingel K (2009) Osteopontin: a fibrosis-related marker molecule in cardiac remodeling of enterovirus myocarditis in the susceptible host. Circ Res 104:851–859. https://doi.org/10.1161/circresaha.109.193805
Szalay G, Sauter M, Hald J, Weinzierl A, Kandolf R, Klingel K (2006) Sustained nitric oxide synthesis contributes to immunopathology in ongoing myocarditis attributable to interleukin-10 disorders. Am J Pathol 169:2085–2093. https://doi.org/10.2353/ajpath.2006.060350
Tomioka N, Kishimoto C, Matsumori A, Kawai C (1986) Effects of prednisolone on acute viral myocarditis in mice. J Am Coll Cardiol 7:868–872. https://doi.org/10.1016/S0735-1097(86)80349-7
Tschope C, Muller I, Xia Y, Savvatis K, Pappritz K, Pinkert S, Lassner D, Heimesaat MM, Spillmann F, Miteva K, Bereswill S, Schultheiss HP, Fechner H, Pieske B, Kuhl U, Van Linthout S (2017) NOD2 (nucleotide-binding oligomerization domain 2) is a major pathogenic mediator of Coxsackievirus B3-induced myocarditis. Circ Heart Fail 10:12. https://doi.org/10.1161/circheartfailure.117.003870
Vanderheyden M, Paulus WJ, Voss M, Knuefermann P, Sivasubramanian N, Mann D, Baumgarten G (2005) Myocardial cytokine gene expression is higher in aortic stenosis than in idiopathic dilated cardiomyopathy. Heart 91:926–931. https://doi.org/10.1136/hrt.2004.035733
Weber A, Wasiliew P, Kracht M (2010) Interleukin-1 (IL-1) pathway. Sci Signal 3:cm1. https://doi.org/10.1126/scisignal.3105cm1
Zhao S, Wu H, Xia W, Chen X, Zhu S, Zhang S, Shao Y, Ma W, Yang D, Zhang J (2014) Periostin expression is upregulated and associated with myocardial fibrosis in human failing hearts. J Cardiol 63:373–378. https://doi.org/10.1016/j.jjcc.2013.09.013
Acknowledgements
We acknowledge Sandra Bundschuh for excellent technical assistance and Hermann Gram (Novartis Pharma AG, Basel, Switzerland) for providing the murine IL-1β antibody.
Funding
This work was partially funded by the German Research Foundation (DFG) KL 595/2-3 to KK. TE was supported by a MD scholarship (Interdisziplinäres Zentrum für Klinische Forschung (IZKF)-Promotionskolleg) provided by the University Hospital Tübingen.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
KK received the IL-1β antibody as a gift and research funding from Novartis AG. The other authors declare that they have no conflict of interest.
Additional information
Lisa Kraft, Tugs Erdenesukh, Martina Sauter and Karin Klingel takes responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Rights and permissions
About this article
Cite this article
Kraft, L., Erdenesukh, T., Sauter, M. et al. Blocking the IL-1β signalling pathway prevents chronic viral myocarditis and cardiac remodeling. Basic Res Cardiol 114, 11 (2019). https://doi.org/10.1007/s00395-019-0719-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00395-019-0719-0