Abstract
Purpose
Non-alcoholic fatty liver disease (NAFLD) is a complex disease, resulting from a variety of genetic and environmental factors. The aim of this case–control study was to evaluate the effect of selected genetic polymorphisms, nutrition aspects and their interaction on the risk of NAFLD.
Methods
The sample consisted of 134 patients with NAFLD and 217 controls. Disease was diagnosed by liver ultrasound and volunteers were clinically and nutritionally assessed. Food groups were extracted from a 172 food-item FFQ questionnaire. Three genetic polymorphisms were assessed: PNPLA3 rs738409, TM6SF2 rs58542926 and GCKR rs780094.
Results
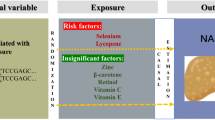
We replicated the effect of previously reported risk factors for NAFLD, such as elevated liver enzymes, obesity and metabolic syndrome. Food groups rich in simple sugars, fat and especially saturated fat were positively associated with NAFLD risk, whereas food groups rich in polyunsaturated fatty acids were reversely associated with the possibility of developing the disease (p < 0.05). Only the PNPLA3 genetic variant was statistically significantly associated with the disease (padditive = 0.015). However, it was found that a one-portion increase in fish intake increased the risk of NAFLD in carriers of the risk allele of TM6SF2 rs58542926 polymorphism compared to non-carriers, after adjusting for age, gender, energy intake, pack-years, PAL, TM6SF2 genotype and fish consumption (ORdominant = 1.503, 95% CI 1.094–2.064).
Conclusions
Fish intake exerts an additive effect on NAFLD risk for carriers of the TM6SF2 polymorphism. This novel finding provides further rationale on the need for personalized nutritional advice, based on the genetic background of NAFLD patients.



Similar content being viewed by others
Abbreviations
- Alb:
-
Albumin
- ALP:
-
Alkaline phosphatase
- ALT:
-
Alanine transaminase
- APAQ:
-
Athens physical activity questionnaire
- AST:
-
Aspartate transaminase
- BMI:
-
Body mass index
- CRP:
-
C-reactive protein
- CVD:
-
Cardiovascular disease
- DBP:
-
Diastolic blood pressure
- Fe:
-
Iron
- Fer:
-
Ferritin
- FGlu:
-
Fasting glucose
- FIns:
-
Fasting insulin
- GERD:
-
Gastroesophageal reflux disease
- GRS:
-
Genetic risk score
- HbA1c:
-
Glycated haemoglobin
- HDL:
-
High-density lipoprotein
- HOMA-IR:
-
Homeostatic model assessment
- IFG:
-
Impaired fasting glucose
- LDL:
-
Low-density lipoprotein
- MetS:
-
Metabolic syndrome
- MUFAs:
-
Monounsaturated fatty acids
- NAFLD:
-
Non-alcoholic fatty liver disease
- NASH:
-
Non-alcoholic steatohepatitis
- NFS:
-
NAFLD fibrosis score
- PLT:
-
Platelets
- PUFAs:
-
Polyunsaturated fatty acids
- SBP:
-
Systolic blood pressure
- SNPs:
-
Single nucleotide polymorphisms
- T2DM:
-
Type 2 diabetes mellitus
- TC:
-
Total cholesterol
- TG:
-
Triglycerides
- WBC:
-
White blood cells
- γ-GT:
-
Gamma-glutamyltransferase
References
Loomba R, Sanyal AJ (2013) The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol 10:686–690
Buzzetti E, Pinzani M, Tsochatzis EA (2016) The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 65:1038–1048
Anstee QM, Day CP (2013) The genetics of NAFLD. Nat Rev Gastroenterol Hepatol 10:645–655
Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, Boerwinkle E, Cohen JC, Hobbs HH (2008) Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet 40:1461–1465
Kozlitina J, Smagris E, Stender S, Nordestgaard BG, Zhou HH, Tybjaerg-Hansen A, Vogt TF, Hobbs HH, Cohen JC (2014) Exome-wide association study identifies a TM6SF2 variant that confers susceptibility to nonalcoholic fatty liver disease. Nat Genet 46:352–356
Mahdessian H, Taxiarchis A, Popov S, Silveira A, Franco-Cereceda A, Hamsten A, Eriksson P, van’t Hooft F (2014) TM6SF2 is a regulator of liver fat metabolism influencing triglyceride secretion and hepatic lipid droplet content. Proc Natl Acad Sci USA 111:8913–8918
Tan HL, Zain SM, Mohamed R, Rampal S, Chin KF, Basu RC, Cheah PL, Mahadeva S, Mohamed Z (2014) Association of glucokinase regulatory gene polymorphisms with risk and severity of non-alcoholic fatty liver disease: an interaction study with adiponutrin gene. J Gastroenterol 49:1056–1064
AISF (Italian Association for the Study of the Liver) (2017) AISF position paper on nonalcoholic fatty liver disease (NAFLD): updates and future directions. Dig Liver Dis, 49, 471–483
Ravi Kanth VV, Sasikala M, Sharma M, Rao PN, Reddy DN (2016) Genetics of non-alcoholic fatty liver disease: From susceptibility and nutrient interactions to management. World J Hepatol 8:827–837
Singh D, Das CJ, Baruah MP (2013) Imaging of non alcoholic fatty liver disease: a road less travelled. Indian J Endocrinol Metab 17:990–995
Friedewald WT, Levy RI, Fredrickson DS (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18:499–502
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419
Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC Jr et al (2009) Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 120:1640–1645
Hyysalo J, Männistö VT, Zhou Y, Arola J, Karja V, Leivonen M, Juuti A, Jaser N, Lallukka S, Kakela P et al (2014) A population-based study on the prevalence of NASH using scores validated against liver histology. J Hepatol 60:839–846
Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, Enders F, Saksena S, Burt AD, Bida JP et al. (2007) The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 45:846–854
Kavouras SA, Maraki MI, Kollia M, Gioxari A, Jansena LT, Sidossis LS (2016) Development, reliability and validity of a physical activity questionnaire for estimating energy expenditure in Greek adults. Sci Sports 31:e47–e53
Dimitriou M, Rallidis LS, Theodoraki EV, Kalafati IP, Kolovou G, Dedoussis GV (2016) Exclusive olive oil consumption has a protective effect on coronary artery disease; overview of the THISEAS study. Public Health Nutr 19:1081–1087
Pan JJ, Fallon MB (2014) Gender and racial differences in nonalcoholic fatty liver disease. World J Hepatol 6:274–283
Mouzaki M, Allard JP (2012) The role of nutrients in the development, progression, and treatment of nonalcoholic fatty liver disease. J Clin Gastroenterol 46:457–467
Parker HM, Johnson NA, Burdon CA, Cohn JS, O’Connor HT, George J (2012) Omega-3 supplementation and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Hepatol, 56, 944–951
Gupta V, Mah XJ, Garcia MC, Antonypillai C, van der Poorten D (2015) Oily fish, coffee and walnuts: Dietary treatment for nonalcoholic fatty liver disease. World J Gastroenterol 21:10621–10635
International HapMap Consortium (2003) The international HapMap project. Nature, 426, 789–796
Petta S, Miele L, Bugianesi E, Camma C, Rosso C, Boccia S, Cabibi D, Di Marco V, Grimaudo S, Grieco A et al (2014) Glucokinase regulatory protein gene polymorphism affects liver fibrosis in non-alcoholic fatty liver disease. PLoS One 9:e87523
Wang X, Liu Z, Wang K, Wang Z, Sun X, Zhong L, Deng G, Song G, Sun B, Peng Z et al (2016) Additive effects of the risk alleles of PNPLA3 and TM6SF2 on non-alcoholic fatty liver disease (NAFLD) in a Chinese population. Front Genet 7:140
Krawczyk M, Rau M, Schattenberg JM, Bantel H, Pathil A, Demir M, Kluwe J, Boettler T, Lammert F, Geier A; NAFLD Clinical Study Group (2017) Combined effects of the PNPLA3 rs738409, TM6SF2 rs58542926, and MBOAT7 rs641738 variants on NAFLD severity: a multicenter biopsy-based study. J Lipid Res 58:247–255
Luukkonen PK, Zhou Y, Nidhina Haridas PA, Dwivedi OP, Hyötyläinen T, Ali A, Juuti A, Leivonen M, Tukiainen T, Ahonen L et al (2017) Impaired hepatic lipid synthesis from polyunsaturated fatty acids in TM6SF2 E167K variant carriers with NAFLD. J Hepatol 67:128–136
O’Hare EA, Yang R, Yerges-Armstrong LM, Sreenivasan U, McFarland R, Leitch CC, Wilson MH, Narina S, Gorden A, Ryan KA et al (2017) TM6SF2 rs58542926 impacts lipid processing in liver and small intestine. Hepatology 65:1526–1542
Ruhanen H, Nidhina Haridas PA, Eskelinen EL, Eriksson O, Olkkonen VM, Käkelä R (2017) Depletion of TM6SF2 disturbs membrane lipid composition and dynamics in HuH7 hepatoma cells. Biochim Biophys Acta 1862:676–685
Scorletti E, West AL, Bhatia L, Hoile SP, McCormick KG, Burdge GC, Lillycrop KA, Clough GF, Calder PC, Byrne CD (2015) Treating liver fat and serum triglyceride levels in NAFLD, effects of PNPLA3 and TM6SF2 genotypes: results from the WELCOME trial. J Hepatol 63:1476–1483
Acknowledgements
This study was funded by the project “Obesity and metabolic syndrome: dietary intervention with Greek raisins in NAFLD/NASH. Investigation of molecular mechanisms” reviewed and approved by the Greek Secretariat for Research and Technology (Cooperation 890/2009).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No potential conflicts of interest relevant to this article were reported.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kalafati, I.P., Dimitriou, M., Borsa, D. et al. Fish intake interacts with TM6SF2 gene variant to affect NAFLD risk: results of a case–control study. Eur J Nutr 58, 1463–1473 (2019). https://doi.org/10.1007/s00394-018-1675-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-018-1675-4