Abstract
Purpose
The present study was designed to determine the effects of both choline form and availability on maternal immune function during lactation.
Methods
Sprague–Dawley rats were randomized to one of the three diets 24–48 h before parturition and fed ad libitum until 21 days postnatal: 1 g/kg choline as free choline (C, n = 11), the current form, and amount of choline in commercial diets; 1 g/kg choline as phosphatidylcholine (PC1, n = 11); or 2.5 g/kg choline as PC (PC2.5, n = 8). Choline metabolites in offspring stomach contents were quantified. At 21 days, lymphocytes from mothers’ mesenteric lymph nodes and spleens were isolated and phenotypes and ex vivo cytokine production after mitogen exposure were determined.
Results
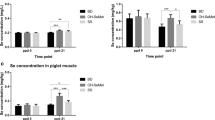
There was a higher proportion of choline and a lower proportion of lyso-PC in stomach contents (representing dam’s milk) of C pups compared to PC1. In the mesenteric lymph nodes, feeding PC1 compared to C led to a higher IL-2 production after Concanavalin A (ConA) stimulation and a higher proportion of T cells (CD3+) and a lower proportion of B cells [immunoglobulin (Ig)κ, CD45RA+, and IgM+; P < 0.05]. Splenocytes from the PC1 group produced more IL-6 and TNF-α after lipopolysaccharides stimulation compared to C (P < 0.05). Splenocytes from the PC2.5 group produced more IL-2 and IL-6 after ConA stimulation compared to PC1 (P < 0.05).
Conclusions
Feeding choline as PC in the maternal diet improved the ability of immune cells to respond ex vivo to mitogens and increasing the amount of PC in the diet further improved T cell proliferation.


Similar content being viewed by others
References
Dellschaft NS, Ruth MR, Goruk S et al (2015) Choline is required in the diet of lactating dams to maintain maternal immune function. Br J Nutr. doi:10.1017/S0007114515001221
da Silva RP, Kelly KB, Lewis ED et al (2015) Choline deficiency impairs intestinal lipid metabolism in the lactating rat. J Nutr Biochem 26:1077–1083. doi:10.1016/j.jnutbio.2015.04.015
Cordero P, Milagro FI, Campion J, Martinez JA (2014) Supplementation with methyl donors during lactation to high-fat-sucrose-fed dams protects offspring against liver fat accumulation when consuming an obesogenic diet. J Dev Orig Health Dis 5:385–395. doi:10.1017/S204017441400035X
Schulz KM, Pearson JN, Gasparrini ME et al (2014) Dietary choline supplementation to dams during pregnancy and lactation mitigates the effects of in utero stress exposure on adult anxiety-related behaviors. Behav Brain Res 268:104–110. doi:10.1016/j.bbr.2014.03.031
Yonemori KM, Lim U, Koga KR et al (2013) Dietary choline and betaine intakes vary in an adult multiethnic population. J Nutr 143:894–899. doi:10.3945/jn.112.171132
Lewis ED, Subhan FB, Bell RC et al (2014) Estimation of choline intake from 24 h dietary intake recalls and contribution of egg and milk consumption to intake among pregnant and lactating women in Alberta. Br J Nutr 112:112–121. doi:10.1017/S0007114514000555
Cheng W-L, Holmes-McNary MQ, Mar M-H et al (1996) Bioavailability of choline and choline esters from milk in rat pups. J Nutr Biochem 7:457–464. doi:10.1016/0955-2863(96)00079-4
Caudill MA (2010) Pre- and postnatal health: evidence of increased choline needs. J Am Diet Assoc 110:1198–1206. doi:10.1016/j.jada.2010.05.009
Bidulescu A, Chambless LE, Siega-Riz AM et al (2007) Usual choline and betaine dietary intake and incident coronary heart disease: the Atherosclerosis Risk in Communities (ARIC) Study. BMC Cardiovasc Disord 7:20. doi:10.1186/1471-2261-7-20
Gossell-Williams M, Fletcher H, McFarlane-Anderson N et al (2005) Dietary intake of choline and plasma choline concentrations in pregnant women in Jamaica. West Indian Med J 54:355–359
Mygind VL, Evans SE, Peddie MC et al (2013) Estimation of usual intake and food sources of choline and betaine in New Zealand reproductive age women. Asia Pac J Clin Nutr 22:319–324
Masih SP, Plumptre L, Ly A et al (2015) Pregnant Canadian women achieve recommended intakes of one-carbon nutrients through prenatal supplementation but the supplement composition, including choline, requires reconsideration. J Nutr 145:1824–1834. doi:10.3945/jn.115.211300
Vennemann FBC, Ioannidou S, Valsta LM et al (2015) Dietary intake and food sources of choline in European populations. Br J Nutr 114:2046–2055. doi:10.1017/S0007114515003700
Reeves PG, Nielsen FH, Fahey GC Jr (1993) AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr 123:1939–1951
Zeisel SH, Mar MH, Zhou Z, da Costa KA (1995) Pregnancy and lactation are associated with diminished concentrations of choline and its metabolites in rat liver. J Nutr 125:3049–3054
Lewis ED, Richard C, Goruk S et al (2016) The form of choline in the maternal diet affects immune development in suckled rat offspring. J Nutr 146:823–830. doi:10.3945/jn.115.225888
Kawashima K, Fujii T, Moriwaki Y, Misawa H (2012) Critical roles of acetylcholine and the muscarinic and nicotinic acetylcholine receptors in the regulation of immune function. Life Sci 91:1027–1032. doi:10.1016/j.lfs.2012.05.006
James SJ, Yin L (1989) Diet-induced DNA damage and altered nucleotide metabolism in lymphocytes from methyl-donor-deficient rats. Carcinogenesis 10:1209–1214
Anderson KE, Whitlon DS, Mueller GC (1985) Role of fatty acid structure in the reversible activation of phosphatidylcholine synthesis in lymphocytes. Biochim Biophys Acta BBA Lipids Lipid Metab 835:360–368. doi:10.1016/0005-2760(85)90292-9
Courrèges MC, Benencia F, Uceda A, Monserrat AJ (2003) Effect of dietary choline deficiency on immunocompetence in Wistar rats. Nutr Res 23:519–526. doi:10.1016/S0271-5317(02)00544-4
Kaplan BJ, Giesbrecht GF, Leung BMY et al (2014) The Alberta Pregnancy Outcomes and Nutrition (APrON) cohort study: rationale and methods. Matern Child Nutr 10:44–60. doi:10.1111/j.1740-8709.2012.00433.x
Field CJ, Thomson CA, Van Aerde JE et al (2000) Lower proportion of CD45R0+ cells and deficient interleukin-10 production by formula-fed infants, compared with human-fed, is corrected with supplementation of long-chain polyunsaturated fatty acids. J Pediatr Gastroenterol Nutr 31:291–299
Blewett HJ, Gerdung CA, Ruth MR et al (2009) Vaccenic acid favourably alters immune function in obese JCR: LA-cp rats. Br J Nutr 102:526. doi:10.1017/S0007114509231722
Xiong Y, Zhao Y-Y, Goruk S et al (2012) Validation of an LC–MS/MS method for the quantification of choline-related compounds and phospholipids in foods and tissues. J Chromatogr B 911:170–179. doi:10.1016/j.jchromb.2012.10.038
Lewis ED, Goruk S, Richard C et al (2016) Feeding a diet devoid of choline to lactating rodents restricts growth and lymphocyte development in offspring. Br J Nutr 116:1001–1012. doi:10.1017/S0007114516002919
Parameswaran N, Patial S (2010) Tumor necrosis factor-alpha signaling in macrophages. Crit Rev Eukaryot Gene Expr. doi:10.1615/CritRevEukarGeneExpr.v20.i2.10
Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S (2011) The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta BBA Mol Cell Res 1813:878–888. doi:10.1016/j.bbamcr.2011.01.034
Tian Y, Pate C, Andreolotti A et al (2008) Cytokine secretion requires phosphatidylcholine synthesis. J Cell Biol 181:945–957. doi:10.1083/jcb.200706152
Couper KN, Blount DG, Riley EM (2008) IL-10: the master regulator of immunity to infection. J Immunol 180:5771–5777. doi:10.4049/jimmunol.180.9.5771
Wieland E, Shipkova M (2016) Lymphocyte surface molecules as immune activation biomarkers. Clin Biochem 49:347–354. doi:10.1016/j.clinbiochem.2015.07.099
Dwyer JM, Johnson C (1981) The use of concanavalin A to study the immunoregulation of human T cells. Clin Exp Immunol 46:237–249
Wei S, Kryczek I, Zou W (2006) Regulatory T-cell compartmentalization and trafficking. Blood 108:426–431. doi:10.1182/blood-2006-01-0177
Brewer JW (2013) Phospholipids: “greasing the wheels” of humoral immunity. Biochim Biophys Acta BBA Mol Cell Biol Lipids 1831:642–651. doi:10.1016/j.bbalip.2012.09.018
Prokazova N, Zvezdina N, Korotaeva A (1998) Effect of lysophosphatidylcholine on transmembrane signal transduction. Biochem Mosc 63:31–37
Noga AA, Vance DE (2003) Insights into the requirement of phosphatidylcholine synthesis for liver function in mice. J Lipid Res 44:1998–2005. doi:10.1194/jlr.M300226-JLR200
Jiang X, Bar HY, Yan J et al (2012) Pregnancy induces transcriptional activation of the peripheral innate immune system and increases oxidative DNA damage among healthy third trimester pregnant women. PLoS One 7:e46736. doi:10.1371/journal.pone.0046736
Rohlfs E, Garner S, Mar M-H, Zeisel SH (1993) Glycerophosphocholine and phosphocholine are the major choline metabolites in rat milk. J Nutr 123:1762–1768
Davenport C, Yan J, Taesuwan S et al (2015) Choline intakes exceeding recommendations during human lactation improve breast milk choline content by increasing PEMT pathway metabolites. J Nutr Biochem 26:903–911. doi:10.1016/j.jnutbio.2015.03.004
Fischer LM, da Costa K-A, Kwock L et al (2010) Dietary choline requirements of women: effects of estrogen and genetic variation. Am J Clin Nutr 92:1113–1119. doi:10.3945/ajcn.2010.30064
Ilcol Y, Ozbek R, Hamurtekin E, Ulus I (2005) Choline status in newborns, infants, children, breast-feeding women, breast-fed infants and human breast milk. J Nutr Biochem 16:489–499. doi:10.1016/j.jnutbio.2005.01.011
Ozarda Y, Cansev M, Ulus IH (2014) Breast milk choline contents are associated with inflammatory status of breastfeeding women. J Hum Lact 30:161–166. doi:10.1177/0890334413508004
Holmes-McNary MQ, Cheng WL, Mar MH et al (1996) Choline and choline esters in human and rat milk and in infant formulas. Am J Clin Nutr 64:572–576
Maas C, Franz AR, Shunova A et al (2016) Choline and polyunsaturated fatty acids in preterm infants’ maternal milk. Eur J Nutr. doi:10.1007/s00394-016-1220-2
Acknowledgements
The authors would like to acknowledge the technical assistance of Nicole Coursen, Marnie Newell and Yuan Yuan Zhao. Supported by Natural Science and Engineering Council of Canada (NSERC RGPIN-2016-05649 and 386652) and Quality Food for Health grant from the ALMA, Alberta Innovates Biosolutions and the Egg Farmers of Alberta (2012Q005R). E.D. Lewis was a recipient of the Izaak Walton Killam Memorial Scholarship and the Queen Elizabeth II Graduate Scholarship. C. Richard was a recipient of postdoctoral fellow scholarships from Canadian Institutes of Health Research, Fonds de Recherche en Santé du Québec and Izaak Walton Killam Memorial Postdoctoral Fellowships. R.L. Jacobs is a Canadian Institutes of Health Research New Investigator.
Author information
Authors and Affiliations
Contributions
CJF, RLJ, and JMC designed the study and obtained funding; NSD, EDL, and SG conducted the study; CR and NSD analyzed data and performed statistical analysis; CR, CJF, and NSD wrote the paper; and CJF has primary responsibility for final content. All authors have helped interpret read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that they have no conflict of interest in relation with this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dellschaft, N.S., Richard, C., Lewis, E.D. et al. The dietary form of choline during lactation affects maternal immune function in rats. Eur J Nutr 57, 2189–2199 (2018). https://doi.org/10.1007/s00394-017-1493-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-017-1493-0