Abstract
Introduction
Peripartum cardiomyopathy (PPCM) is an important cause of pregnancy-associated heart failure worldwide. Although a significant number of women recover their left ventricular (LV) function within 12 months, some remain with persistently reduced systolic function.
Methods
Knowledge gaps exist on predictors of myocardial recovery in PPCM. N-terminal pro-brain natriuretic peptide (NT-proBNP) is the only clinically established biomarker with diagnostic value in PPCM. We aimed to establish whether NT-proBNP could serve as a predictor of LV recovery in PPCM, as measured by LV end-diastolic volume (LVEDD) and LV ejection fraction (LVEF).
Results
This study of 35 women with PPCM (mean age 30.0 ± 5.9 years) had a median NT-proBNP of 834.7 pg/ml (IQR 571.2–1840.5) at baseline. Within the first year of follow-up, 51.4% of the cohort recovered their LV dimensions (LVEDD < 55 mm) and systolic function (LVEF > 50%). Women without LV recovery presented with higher NT-proBNP at baseline. Multivariable regression analyses demonstrated that NT-proBNP of ≥ 900 pg/ml at the time of diagnosis was predictive of failure to recover LVEDD (OR 0.22, 95% CI 0.05–0.95, P = 0.043) or LVEF (OR 0.20 [95% CI 0.04–0.89], p = 0.035) at follow-up.
Conclusions
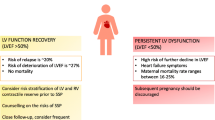
We have demonstrated that NT-proBNP has a prognostic value in predicting LV recovery of patients with PPCM. Patients with NT-proBNP of ≥ 900 pg/ml were less likely to show any improvement in LVEF or LVEDD. Our findings have implications for clinical practice as patients with higher NT-proBNP might require more aggressive therapy and more intensive follow-up. Point-of-care NT-proBNP for diagnosis and risk stratification warrants further investigation.
Similar content being viewed by others
Introduction
Peripartum cardiomyopathy (PPCM) is an important cause of pregnancy-associated heart failure and occurs in women towards the end of pregnancy or within the first five months after delivery [1, 2]. Although up to 46% of patients with PPCM recover their left ventricular (LV) function within 6 months, 23% remain with severely impaired LV systolic function and develop chronic heart failure [3].
Despite recent advances in the management of PPCM, predictors of myocardial recovery remain poorly understood. Previous baseline clinical factors that have been shown to influence LV recovery include LVEF, [4,5,6] LV dimensions, [5, 7, 8] presence of LV thrombus, [7] right ventricular (RV) systolic dysfunction [9] and African-American ethnicity [5, 7, 10, 11]. Identification of predictors of LV recovery could help to risk stratify patients at the time of PPCM diagnosis, and identify those patients that may benefit from more intensive therapy and follow-up.
B-type natriuretic peptide (BNP) and its prohormone, N-terminal B-type natriuretic peptide (NT-proBNP), are released in response to cardiac wall stress, [12, 13] and are important biomarkers in the diagnosis of heart failure [14]. However, the role of natriuretic peptides is predominantly to rule out heart failure. Previous studies have shown that NT-proBNP is elevated at the time of diagnosis of PPCM [15, 16] and a diagnosis of PPCM is unlikely if a patient presents with BNP < 100 pg/ml or NT-proBNP < 300 pg/ml [2]. Furthermore, NT-proBNP is useful in differentiating healthy postpartum women from those with PPCM or pre-eclampsia [6, 15, 17].
While elevated NT-proBNP levels have been shown to predict mortality and cardiovascular events in patients with heart failure, even amongst those who were asymptomatic, [18] little is known about the prognostic value of NT-proBNP amongst patients with PPCM. In this study, we aimed to assess whether NT-proBNP could serve as predictor of LV recovery in PPCM.
Methods
Study design and recruitment
Women with PPCM, seen at the dedicated Cardiomyopathy Clinic at Groote Schuur Hospital, were recruited between 2013 and 2018. Patients were referred from primary or secondary care facilities or within the tertiary/quaternary hospital, and assessed by a team of cardiologists and heart failure specialists.
Inclusion criteria included: (1) primary diagnosis of PPCM, i.e. documented clinical evidence of LV systolic dysfunction towards the end of pregnancy or during the first five months postpartum; (2) no other identifiable causes of heart failure; (3) LVEF ≤ 45% on presentation confirmed by transthoracic echocardiography. Exclusion criteria were: (1) patient unable to give informed consent; (2) other identifiable causes of heart failure; and (3) patients younger than 18 years.
This study was approved by the University of Cape Town’s Faculty of Health Sciences Human Research Ethics Committee (HREC ref no R033/2013), and complied with the Declaration of Helsinki. All patients provided written informed consent prior to study entry.
Eligible patients were enrolled at the baseline visit, at which time their medical and obstetric history, New York Heart Association (NYHA) functional class, clinical examination findings and prescribed medication were recorded. All patients had 12-lead electrocardiogram (ECG) and echocardiogram at the baseline visit. Blood was collected at the baseline visit for the measurement of full blood count, renal function and NT-proBNP.
12-lead ECG
A resting 12-lead ECG was performed for all patients at baseline using a MAC 5500 HD (GE Healthcare, Chicago, Illinois, USA) machine. The ECG was analysed for heart rate, rhythm, QRS duration, LV hypertrophy (LVH) by Sokolow–Lyon criteria [19], and a QT interval measurement, corrected by Bazett’s formula (QTcB) [20]. Sinus tachycardia was defined as a heart rate of ≥ 100 beats per minute (bpm). A QTcB interval of ≥ 460 ms was regarded as prolonged [21].
Echocardiographic assessment
Echocardiography was performed at the time of diagnosis and at follow-up. Two-dimensional and targeted M-mode echocardiography with Doppler colour flow mapping were performed using either a Philips CX50 (Philips, Amsterdam, Netherlands) or a VIVIDi (General Electric Company, Fairfield, Connecticut, USA) echocardiography machine. LV dimensions (i.e. LV end-diastolic diameter [LVEDD] and LV end-systolic diameter [LVESD]) and global systolic function (LV ejection fraction [LVEF]) were measured according to the guidelines endorsed by the American Society of Echocardiography [22].
Blood tests
Blood samples were taken at the baseline visit. Full blood count and renal function were analysed by the National Health Laboratory Service (NHLS) at Groote Schuur Hospital. For NT-proBNP testing, plasma or serum was separated by centrifugation and aliquots were stored at − 80 °C. NT-proBNP was measured for each participant using the BNP Fragment EIA kit (Biomedica; Vienna, Austria). NT-proBNP values were converted to pg/ml as recommended by current clinical practice guidelines.
Outcome
We considered two separate echocardiographic measures for LV recovery, i.e. LVEDD < 55 mm and LVEF ≥ 50% within the 12-month follow-up period. Patients who did not fulfil these echocardiographic criteria at follow-up echocardiogram, and those who died within the study period, were considered to have no LV recovery.
Statistical analysis
Data were collected on Research Electronic Data Capture (REDCap Version 9.5.13), a secure electronic database hosted by the University of Cape Town [23], before being exported to Stata (Version 14.2, StataCorp, College Station, TX, USA) for statistical analysis. Descriptive statistics were used to summarise data. Distribution of data was determined by Shapiro–Wilk test. Continuous variables were summarised as means with standard deviations (SD) for parametric data or median with interquartile range (IQR) for non-parametric data. Categorical variables were expressed as frequencies and percentages. Considering the median NT-proBNP at baseline, we used a cut-off value of 900 pg/ml as a dichotomous variable according to which patients were stratified. Where appropriate, we used a Kruskal–Wallis or Wilcoxon rank-sum test (for continuous variables) and chi-squared or Fisher’s exact test (for categorical variables), to compare outcome measures at follow-up, and whether patients had an initial NT-proBNP of ≥ or < 900 pg/ml. Univariable and multivariable logistic regression analyses were done to determine the association between NT-proBNP value of ≥ 900 pg/ml at presentation and LV recovery (i.e. LV dimension and systolic function) at follow-up. NT-proBNP was adjusted for age and BMI. A p value of < 0.05 was considered to indicate statistical significance.
Results
This cohort of 35 women with PPCM had a mean age of 30.0 ± 5.9 years (Table 1). Almost half of the cohort (45.7%) was multiparous (parity > 3). At the time of diagnosis, 40% had a NYHA functional class III or IV. Overall, the patients presented with a mean heart rate of 90.6 ± 19.6 bpm and a median systolic and diastolic blood pressure of 112 mmHg (IQR 105—138) and 76 mmHg (IQR 70—85), respectively. On echocardiography, the median LVEF was 31% (IQR 24—39), with an LVEDD of 58 mm (IQR 53—64) and LA diameter of 35 mm (IQR 33—39). The median NT-proBNP at the time of diagnosis was 834.7 pg/ml (IQR 571.2—1840.5). The median Hb was 11.9 g/dL (IQR 9.9—12.9), and there was no renal impairment. By the time of discharge, heart failure therapy consisted of beta-blocker (94.3%), angiotensin converting enzyme (ACE)-inhibitor or angiotensin receptor blocker (ARB) (80%), mineralocorticoid-receptor antagonist (MRA) (45.7%) and loop diuretics (91.4%). The dopamine agonist, bromocriptine, was prescribed to 41.1% of the patients in this cohort.
Recovery of LV dimensions
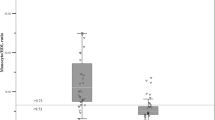
Figure 1 shows that most women with PPCM showed overall improvement in LV dimensions at follow-up. However, 18 women (51.4%) recovered their LV dimensions (LVEDD < 55 mm) within the first year after diagnosis. As depicted in Table 1, women who showed recovery of LV dimensions, had a significantly lower heart rate (83 ± 15 vs 98 ± 22 bpm, p = 0.025) and were less likely to have sinus tachycardia (11 vs 59%, p = 0.012) at initial presentation. The baseline QTc interval was longer, however, in patients who failed to recover their LV dimensions (470 [IQR 453—480] vs. 443 ms [IQR 414—456], p = 0.030). The baseline LVEF did not predict recovery of LV dimensions. The initial NT-proBNP was lower in those with recovery of LV dimensions within 12 months (622.3 [IQR 534—1000.7] vs. 1020.1 pg/ml [IQR 713.4—1865.4], p = 0.041). As depicted in Fig. 1a and b, the LVEDD decreased at follow visits, regardless of whether the initial NT-proBNP level measured ≥ or < 900 pg/ml (OR 0.42 [95% 0.06—2.95], p = 0.380), though the gradient of decline was greater in those with NT-proBNP level measured < 900 pg/ml at baseline. Women with an NT-proBNP of ≥ 900 pg/ml had significantly higher LVEDD at follow-up (58 ± 6.66 vs 49.9 ± 5.05 mm, p < 0.001). As shown in Table 2, multivariable regression analysis found that an NT-proBNP of ≥ 900 pg/ml at the time of PPCM diagnosis was predictive of failure to achieve an LVEDD within normal range (LVEDD < 55 mm) at follow-up (OR 0.22 [95% CI 0.05–0.95] p = 0.043).
Recovery of LV systolic function
Within the first year of follow-up, 51.4% of the cohort recovered their systolic function (LVEF > 50%). Women who did not recover systolic function by 12 months were more likely to present with a NYHA functional class III or IV (58.8 vs. 22.2%; p = 0.041) and sinus tachycardia (52.9 vs. 11.1%; p = 0.012) at the time of first diagnosis. There was no significant difference in haemoglobin, creatinine or hs-CRP at baseline between women with LV recovery and LV non-recovery.
Although not statistically significant, there was a notable difference in the initial NT-proBNP values between those who recovered their LV function by 12 months and those who did not (693.2 pg/ml [IQR 543.9—1000.7] vs. 1066.2 pg/ml [682.5—1962], p = 0.065). More than two-thirds of those who did not recover their LV function within one year presented initially with NT-proBNP levels of ≥ 900 pg/m (68.4 vs. 31.3%, p = 0.028). Patients with a baseline NT-proBNP < 900 pg/ml showed more consistent recovery of their systolic function at follow-up (Fig. 1c) (LVEF of 53%, IQR 49–56), whereas there was more variation in LVEF at follow-up amongst those patients with NT-proBNP ≥ 900 pg/ml (LVEF of 42%, IQR 31–51, p = 0.045) (Fig. 1d). Indeed, women with NT-proBNP levels of ≥ 900 pg/ml had a lower likelihood of LV functional improvement (OR 0.12 [95% CI 0.02—0.69], p = 0.017). As depicted in Table 2, multivariable regression analysis found NT-proBNP level of ≥ 900 pg/ml to be predictive of persistent impaired systolic function (LVEF < 50%) at follow-up (OR 0.20 [95% CI 0.04—0.89], p = 0.035).
Factors associated with increased NT-proBNP
When adjusted for age and BMI, an NT-proBNP level of ≥ 900 pg/ml remained a significant predictor of recovery of LV dimension (OR 0.22 [95% CI 0.05—0.95], p = 0.043) and systolic function (OR 0.20 [95% CI 0.04—0.89], p = 0.035) within 12 months (Table 2).
There was no correlation between NT-proBNP and blood pressure (SBP r = – 0.06, p = 0.751; DBP r = -0.127, p = 0.467) – Fig. 2. Similarly, NT-proBNP did not correlate with BMI or creatinine. However, there was a modest correlation between NT-proBNP levels and heart rate (r = 0.344, p = 0.043) and LVEDD (r = 0.420; p = 0.012).
Table 3 depicts the clinical factors that were associated with higher baseline NT-proBNP levels. Women with NT-proBNP ≥ 900 pg/ml at the time of diagnosis, had a higher heart rate (98 vs 85 bpm; p = 0.028), a tendency towards a larger left atrial (LA) diameter (37 vs 35 mm, p = 0.091), and significantly increased LVEDD (62.5 vs 55 mm; p = 0.027). Furthermore, these patients presented with a significantly lower LVEF (26 vs 35%; p = 0.035) at the time of diagnosis. There was no difference in parameters that are known to influence NT-proBNP (i.e. BMI, creatinine).
Discussion
In this study, we show that women with PPCM present with markedly increased NT-pro-BNP levels at the time of diagnosis. In this regard, a baseline NT-proBNP ≥ 900 pg/ml is predictive of failure to recover LV systolic function and dimensions at one-year follow-up. Importantly, NT-proBNP levels are usually not elevated during normal pregnancy or healthy postpartum period [24]. NT-proBNP levels in our cohort were higher than what has been reported for women during normal pregnancy and healthy postpartum period, [15, 24] and those reported for women with pre-eclampsia [17]. This corresponds to what has previously been reported for patients with PPCM in South Africa, Germany and China [6, 15, 16]. The NT-proBNP values seen in this cohort (mean 834.7 pg/ml), however, were lower than those recently reported for the 739 patients included in the European Observational Research Project (EORP) on PPCM, where the median NT-proBNP was 3308 pg/ml [3].
Contemporary heart failure guidelines recommend natriuretic peptides as the biomarker of choice in diagnostic work-up of patients with heart failure [25]. In this regard, the diagnostic role of BNP or NT-proBNP is predominantly to exclude heart failure in peripartum patients. In a study by Malhame et al., a BNP cut-off value of < 111 pg/ml excluded heart failure in pregnant and postpartum women [26]. For PPCM, a threshold of < 100 pg/ml for BNP and < 300 pg/ml for NT-proBNP was proposed to rule out heart failure during pregnancy or the postpartum period [27].
Natriuretic peptides have also been studied for its prognostic value during pregnancy and the postpartum period. Normal BNP levels (< 100 pg/ml) have been shown to have a negative predictive value for adverse maternal events in pregnant women with heart disease [24]. In women with congenital heart disease, BNP levels > 128 pg/ml measured at 20-week gestation predicted adverse cardiovascular events later in pregnancy [28].
The predictive value of NT-proBNP in patients with mild to moderate heart failure was studied in a sub-study of the COPERNICUS trial (n = 1,011) in which patients were stratified according to whether their NT-proBNP levels were above or below the median value of the cohort [29]. Hartmann et al. described increased mortality and rehospitalisation for heart failure amongst patients with an NT-proBNP above the median of the cohort [29]. Considering the median baseline NT-proBNP in this cohort, we chose an arbitrary cut-off value of 900 pg/ml according to which our patients were classified. We found that patients with NT-pro-BNP of ≥ 900 pg/ml had significantly higher LVEDD and significantly lower LVEF at one-year follow-up. Although most patients showed some improvement in LVEDD and LVEF at follow-up, women with NT-pro-BNP of ≥ 900 pg/ml were less likely to recover their LV dimensions and systolic function within normal range within one-year follow-up.
When interpreting NT-proBNP levels in heart failure, various clinical factors need to be considered. The level of natriuretic peptides increases with age, and therefore, higher cut-off values are suggested for the elderly. Whereas obesity lowers the concentration of natriuretic peptides, renal disease and atrial arrhythmias (atrial fibrillation (AF) in particular) are associated with higher NT-proBNP levels. None of the patients in this cohort had AF; this was not surprising, as AF has previously been described to be rare in PPCM [30]. Furthermore, the median creatinine was 61 µmol/L in this cohort and there was no significant correlation between creatinine and NT-proBNP. Adjusting for age and BMI, baseline NT-proBNP ≥ 900 pg/ml was a predictor of failure to recover LV dimensions and systolic function within one year in this South African cohort. In a Chinese cohort of 71 patients with PPCM, Li et al. reported that NT-proBNP levels of > 1860 pg/ml were associated with a threefold increase in persistent LV systolic dysfunction at follow-up [16]. In contrast, Biteker et al. did not find BNP to be predictive of recovery of LV dimensions and systolic function in a cohort of 43 women with PPCM from Turkey [31].
Although our study showed that in most patients, there was an improvement in LV dimensions and systolic function, some remained with an LVEDD > 55 mm and LVEF < 50% at follow-up. We found that women with baseline NT-proBNP ≥ 900 pg/ml were more likely to show no improvement in their LV systolic function at one-year follow-up. This is supported by previous work by Forster et al. who reported that NT-proBNP levels were significantly higher in those women who did not show an improvement of at least 10 percentage units (e.g. 25–35%). In their study, NT-proBNP levels remained significantly higher in those who did not improve their LV function [15].
Inflammation, increased levels of oxidative stress and systemic angiogenic imbalance appear to play a crucial role in the pathophysiology of PPCM. Through unknown mechanisms, increased levels of oxidative stress cause a cleavage of prolactin into a 16-kDa fragment, which causes endothelial dysfunction and induces cardiomyocyte apoptosis [32, 33]. Moreover, auto-immune mechanisms have been suggested to be involved in the pathogenesis of PPCM [34, 35]. More severe forms of the disease have been shown to have higher levels of auto-antibodies. In this regard, circulating auto-antibodies against cardiac sarcomeric myosin (MHC) and troponin I (TnI) were also associated with higher levels of NT-proBNP. Indeed, patients with auto-antibodies have been described to have lower rates of LV recovery [35].
Increased NT-proBNP levels are associated with LV remodeling [36]. In this cohort, elevated levels of NT-proBNP tended to be associated with a lower LVEF. However, there was there was a significant correlation between LVEDD and NT-proBNP in this cohort. As expected, those with NYHA functional class III or IV also had higher levels of NT-proBNP, in keeping with a more severe stage of heart failure. Although there was no correlation between systolic or diastolic blood pressure and NT-proBNP, we found a positive correlation between heart rate and NT-proBNP levels. Those patients with sinus tachycardia at time of initial diagnosis, had significantly higher levels of NT-proBNP. This underlines the importance of recognising the presence of sinus tachycardia in patients with heart failure, as sinus tachycardia has previously been reported as a predictor of poor outcome in PPCM [37].
Although not explored in this study, point-of-care (POC) NT-proBNP tests have previously been used in patients with heart failure. The benefit of POC testing is its ease of use, affordability and the immediate availability of the result [38]. Therefore, NT-proBNP bedside testing in patients with PPCM is an exciting prospect, especially in health care centres in low- and middle-income countries, where echocardiography is not readily available [14]. POC NT-proBNP testing may potentially assist the primary care physicians with diagnosis of PPCM, risk stratification, and timely referral [39]. The role of POC NT-proBNP testing, however, still needs to be studied in PPCM.
Limitations
As this is a single-centre study conducted at a tertiary hospital, where the most severe cases of PPCM are generally seen, there is a possibility of referral bias. Furthermore, considering that PPCM is a rare disease, we acknowledge that the small sample size of this study might have affected the precision of estimates, especially in the logistic regression analyses. We therefore encourage validation of our findings in a larger, multi-centred cohort.
This study also lacks a control group and NT-proBNP measurements at follow-up. The slope of change in NT-proBNP levels in patients with PPCM might provide important information regarding the changes of LV recovery and selection of correct heart failure treatment in future studies.
We acknowledge that RV function, which has previously been shown to have important prognostic value in PPCM, [9] was not assessed in this study. Future studies should evaluate the impact of RV size and function on NT-pro-BNP levels in PPCM.
Conclusions
We demonstrate the prognostic value of NT-pro-BNP for LV recovery in PPCM. We have shown that NT-proBNP may be useful in the risk stratification of patients with PPCM and may be used to implement more intensive treatment strategies of patients who have an NT-proBNP ≥ 900 pg/ml at diagnosis and individualise follow-up regimens. The application of POC NT-proBNP testing should be further studied for its use of diagnosis and risk stratification for patients with PPCM.
References
Sliwa K, Hilfiker-Kleiner D, Petrie MC, Mebazaa A, Pieske B, Buchmann E et al (2010) Current state of knowledge on aetiology, diagnosis, management, and therapy of peripartum cardiomyopathy: a position statement from the Heart Failure Association of the European Society of Cardiology Working Group on peripartum cardiomyopathy. Eur J Heart Fail 12(8):767–778
Bauersachs J, Konig T, van der Meer P, Petrie MC, Hilfiker-Kleiner D, Mbakwem A et al (2019) Pathophysiology, diagnosis and management of peripartum cardiomyopathy: a position statement from the Heart Failure Association of the European Society of Cardiology Study Group on peripartum cardiomyopathy. Eur J Heart Fail 21(7):827–843
Sliwa K, Petrie MC, van der Meer P, Mebazaa A, Hilfiker-Kleiner D, Jackson AM et al (2020) Clinical presentation, management, and 6-month outcomes in women with peripartum cardiomyopathy: an ESC EORP registry. Eur Heart J. https://doi.org/10.1093/eurheartj/ehaa455
Goland S, Bitar F, Modi K, Safirstein J, Ro A, Mirocha J et al (2011) Evaluation of the clinical relevance of baseline left ventricular ejection fraction as a predictor of recovery or persistence of severe dysfunction in women in the United States with peripartum cardiomyopathy. J Card Fail 17(5):426–430
McNamara DM, Elkayam U, Alharethi R, Damp J, Hsich E, Ewald G et al (2015) Clinical outcomes for peripartum cardiomyopathy in North America: results of the IPAC Study (Investigations of pregnancy-associated cardiomyopathy). J Am Coll Cardiol 66(8):905–914
Haghikia A, Podewski E, Libhaber E, Labidi S, Fischer D, Roentgen P et al (2013) Phenotyping and outcome on contemporary management in a German cohort of patients with peripartum cardiomyopathy. Basic Res Cardiol 108(4):366
Amos AM, Jaber WA, Russell SD (2006) Improved outcomes in peripartum cardiomyopathy with contemporary. Am Heart J 152(3):509–513
Blauwet LA, Libhaber E, Forster O, Tibazarwa K, Mebazaa A, Hilfiker-Kleiner D et al (2013) Predictors of outcome in 176 South African patients with peripartum cardiomyopathy. Heart 99(5):308–313
Haghikia A, Rontgen P, Vogel-Claussen J, Schwab J, Westenfeld R, Ehlermann P et al (2015) Prognostic implication of right ventricular involvement in peripartum cardiomyopathy: a cardiovascular magnetic resonance study. ESC Heart Fail 2(4):139–149
Irizarry OC, Levine LD, Lewey J, Boyer T, Riis V, Elovitz MA et al (2017) Comparison of clinical characteristics and outcomes of peripartum cardiomyopathy between african american and non-african american women. JAMA Cardiol 2(11):1256–1260
Goland S, Modi K, Hatamizadeh P, Elkayam U (2013) Differences in clinical profile of African-American women with peripartum cardiomyopathy in the United States. J Card Fail 19(4):214–218
Mueller C, McDonald K, de Boer RA, Maisel A, Cleland JGF, Kozhuharov N et al (2019) Heart failure association of the european society of cardiology practical guidance on the use of natriuretic peptide concentrations. Eur J Heart Fail 21(6):715–731
Santaguida PL, Don-Wauchope AC, Oremus M, McKelvie R, Ali U, Hill SA et al (2014) BNP and NT-proBNP as prognostic markers in persons with acute decompensated heart failure: a systematic review. Heart Fail Rev 19(4):453–470
Taylor KS, Verbakel JY, Feakins BG, Price CP, Perera R, Bankhead C et al (2018) Diagnostic accuracy of point-of-care natriuretic peptide testing for chronic heart failure in ambulatory care: systematic review and meta-analysis. BMJ 361:k1450
Forster O, Hilfiker-Kleiner D, Ansari AA, Sundstrom JB, Libhaber E, Tshani W et al (2008) Reversal of IFN-gamma, oxLDL and prolactin serum levels correlate with clinical improvement in patients with peripartum cardiomyopathy. Eur J Heart Fail 10(9):861–868
Li W, Li H, Long Y (2016) Clinical characteristics and long-term predictors of persistent left ventricular systolic dysfunction in peripartum cardiomyopathy. Can J Cardiol 32(3):362–368
Resnik JL, Hong C, Resnik R, Kazanegra R, Beede J, Bhalla V et al (2005) Evaluation of B-type natriuretic peptide (BNP) levels in normal and preeclamptic women. Am J Obstet Gynecol 193(2):450–454
Doust JA, Pietrzak E, Dobson A, Glasziou P (2005) How well does B-type natriuretic peptide predict death and cardiac events in patients with heart failure: systematic review. BMJ (Clin Res ed). 330(7492):625
Sokolow M, Lyon TP (1949) The ventricular complex in left ventricular hypertrophy as obtained by unipolar precordial and limb leads. Am Heart J 37(2):161–186
Bazett HC (1997) An Analysis of the Time-Relations of Electrocardiograms. Ann Noninvasive Electrocardiol 2(2):177–194
Rautaharju PM, Surawicz B, Gettes LS, Bailey JJ, Childers R, Deal BJ, et al. (2009) AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part IV: the ST segment, T and U waves, and the QT interval: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized. Electrocardiology. J Am Coll Cardiol 53(11):982–91
Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L et al (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 28(1):1-39 e14
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG (2009) Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42(2):377–381
Tanous D, Siu SC, Mason J, Greutmann M, Wald RM, Parker JD et al (2010) B-type natriuretic peptide in pregnant women with heart disease. J Am Coll Cardiol 56(15):1247–1253
Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS et al (2016) 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 37(27):2129–2200
Malhame I, Hurlburt H, Larson L, Poppas A, Nau C, Bourjeily G et al (2019) Sensitivity and specificity of B-type natriuretic peptide in diagnosing heart failure in pregnancy. Obstet Gynecol 134(3):440–449
Bauersachs J, Arrigo M, Hilfiker-Kleiner D, Veltmann C, Coats AJ, Crespo-Leiro MG et al (2016) Current management of patients with severe acute peripartum cardiomyopathy: practical guidance from the Heart Failure Association of the European Society of Cardiology Study Group on peripartum cardiomyopathy. Eur J Heart Fail 18(9):1096–1105
Kampman MA, Balci A, van Veldhuisen DJ, van Dijk AP, Roos-Hesselink JW, Sollie-Szarynska KM et al (2014) N-terminal pro-B-type natriuretic peptide predicts cardiovascular complications in pregnant women with congenital heart disease. Eur Heart J 35(11):708–715
Hartmann F, Packer M, Coats AJ, Fowler MB, Krum H, Mohacsi P et al (2004) Prognostic impact of plasma N-terminal pro-brain natriuretic peptide in severe chronic congestive heart failure: a substudy of the Carvedilol Prospective Randomized Cumulative Survival (COPERNICUS) trial. Circulation 110(13):1780–1786
Hoevelmann J, Hahnle L, Hahnle J, Sliwa K, Viljoen C (2020) Detection and management of arrhythmias in peripartum cardiomyopathy. Cardiovasc Diagn Ther 10(2):325–335
Biteker M, Ilhan E, Biteker G, Duman D, Bozkurt B (2012) Delayed recovery in peripartum cardiomyopathy: an indication for long-term follow-up and sustained therapy. Eur J Heart Fail 14(8):895–901
Hilfiker-Kleiner D, Kaminski K, Podewski E, Bonda T, Schaefer A, Sliwa K et al (2007) A cathepsin D-cleaved 16 kDa form of prolactin mediates postpartum cardiomyopathy. Cell 128(3):589–600
Halkein J, Tabruyn SP, Ricke-Hoch M, Haghikia A, Nguyen NQ, Scherr M et al (2013) MicroRNA-146a is a therapeutic target and biomarker for peripartum cardiomyopathy. J Clin Invest 123(5):2143–2154
Liu J, Wang Y, Chen M, Zhao W, Wang X, Wang H et al (2014) The correlation between peripartum cardiomyopathy and autoantibodies against cardiovascular receptors. PLoS One 9(1):e86770
Haghikia A, Kaya Z, Schwab J, Westenfeld R, Ehlermann P, Bachelier K et al (2015) Evidence of autoantibodies against cardiac troponin I and sarcomeric myosin in peripartum cardiomyopathy. Basic Res Cardiol 110(6):60
Daubert MA, Adams K, Yow E, Barnhart HX, Douglas PS, Rimmer S et al (2019) NT-proBNP Goal achievement is associated with significant reverse remodeling and improved clinical outcomes in HFrEF. JACC Heart Fail 7(2):158–168
Hoevelmann J, Viljoen CA, Manning K, Baard J, Hahnle L, Ntsekhe M et al (2019) The prognostic significance of the 12-lead ECG in peripartum cardiomyopathy. Int J Cardiol 276:177–184
Bugge C, Sether EM, Pahle A, Halvorsen S, Sonbo Kristiansen I (2018) Diagnosing heart failure with NT-proBNP point-of-care testing: lower costs and better outcomes A decision analytic study. BJGP Open. 2(3):bjgpopen18X101596
Morbach C, Buck T, Rost C, Peter S, Gunther S, Stork S et al (2018) Point-of-care B-type natriuretic peptide and portable echocardiography for assessment of patients with suspected heart failure in primary care: rationale and design of the three-part Handheld-BNP program and results of the training study. Clin Res Cardiol 107(2):95–107
Funding
Open Access funding enabled and organized by Projekt DEAL. This study was supported by the South African Medical Research Council and National Research Foundation of South Africa.
Author information
Authors and Affiliations
Contributions
JH, CAV and KS designed the study. JH, CAV, FA and EM collected and analysed the data. JH and CAV drafted the manuscript, which was critically revised by EM, SK, JC, OB, MN, NN and KS. All authors agreed on the final manuscript for submission.
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no conflicting interests to disclose.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hoevelmann, J., Muller, E., Azibani, F. et al. Prognostic value of NT-proBNP for myocardial recovery in peripartum cardiomyopathy (PPCM). Clin Res Cardiol 110, 1259–1269 (2021). https://doi.org/10.1007/s00392-021-01808-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-021-01808-z