Abstract
Background
Determination of cardiac output (CO) is essential in diagnosis and management of heart failure (HF). The gold standard to obtain CO is invasive assessment via thermodilution (TD). Noninvasive pulse contour analysis (NPCA) is supposed as a new method of CO determination. However, a validation of this method in HF is pending and performed in the present study.
Methods
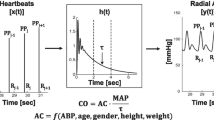
Patients with chronic-stable HF and reduced left ventricular ejection fraction (LVEF ≤ 45%; HF-REF) underwent right heart catheterization including TD. NPCA using the CNAP Monitor (V5.2.14, CNSystems Medizintechnik AG) was performed simultaneously. Three standardized TD measurements were compared with simultaneous auto-calibrated NPCA CO measurements.
Results
In total, 84 consecutive HF-REF patients were enrolled prospectively in this study. In 4 patients (5%), TD was not successful and for 22 patients (26%, 18 with left ventricular assist device), no NPCA signal could be obtained. For the remaining 58 patients, Bland–Altman analysis revealed a mean bias of + 1.92 L/min (limits of agreement ± 2.28 L/min, percentage error 47.4%) for CO. With decreasing cardiac index, as determined by the gold standard of TD, there was an increasing gap between CO values obtained by TD and NPCA (r = − 0.75, p < 0.001), resulting in a systematic overestimation of CO in more severe HF. TD-CI classified 52 (90%) patients to have a reduced CI (< 2.5 L/min/m2), while NPCA documented a reduced CI in 18 patients (31%) only.
Conclusions
In HF-REF patients, auto-calibrated NPCA systematically overestimates CO with decrease in cardiac function. Therefore, to date, NPCA cannot be recommended in this cohort.




Similar content being viewed by others
References
Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P (2016) esc guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 37:2893–2962
Tschöpe C, Birner C, Böhm M, Bruder O, Frantz S, Luchner A, Maier L, Störk S, Kherad B, Laufs U (2017) Heart failure with preserved ejection fraction: current management and future strategies: expert opinion on the behalf of the Nucleus of the “Heart Failure Working Group” of the German Society of Cardiology (DKG). Clin Res Cardiol. https://doi.org/10.1007/s00392-017-1170-6 (Epub ahead of print)
Cotter G, Davison BA, Butler J, Collins SP, Ezekowitz JA, Felker GM, Filippatos G, Levy PD, Metra M, Ponikowski P, Teerlink JR, Voors AA, Senger S, Bharucha D, Goin K, Soergel DG, Pang PS (2017) Relationship between baseline systolic blood pressure and long-term outcomes in acute heart failure patients treated with TRV027: an exploratory subgroup analysis of BLAST-AHF. Clin Res Cardiol. https://doi.org/10.1007/s00392-017-1168-0 (Epub ahead of print)
Nikolaidou T, Pellicori P, Zhang J, Kazmi S, Goode KM, Cleland JG, Clark AL (2017) Prevalence, predictors, and prognostic implications of PR interval prolongation in patients with heart failure. Clin Res Cardiol. https://doi.org/10.1007/s00392-017-1162-6 (Epub ahead of print)
Fu M, Ahrenmark U, Berglund S, Lindholm CJ, Lehto A, Broberg AM, Tasevska-Dinevska G, Wikstrom G, Ågard A, Andersson B, undefined undefined (2017) Adherence to optimal heart rate control in heart failure with reduced ejection fraction: insight from a survey of heart rate in heart failure in Sweden (HR-HF study). Clin Res Cardiol. https://doi.org/10.1007/s00392-017-1146-6 (Epub ahead of print)
Störk S, Handrock R, Jacob J, Walker J, Calado F, Lahoz R, Hupfer S, Klebs S (2017) Treatment of chronic heart failure in Germany: a retrospective database study. Clin Res Cardiol 106:923–932. https://doi.org/10.1007/s00392-017-1138-6
Störk S, Handrock R, Jacob J, Walker J, Calado F, Lahoz R, Hupfer S, Klebs S (2017) Epidemiology of heart failure in Germany: a retrospective database study. Clin Res Cardiol 106:913–922. https://doi.org/10.1007/s00392-017-1137-7
Müller D, Remppis A, Schauerte P, Schmidt-Schweda S, Burkhoff D, Rousso B, Gutterman D, Senges J, Hindricks G, Kuck KH (2017) Clinical effects of long-term cardiac contractility modulation (CCM) in subjects with heart failure caused by left ventricular systolic dysfunction. Clin Res Cardiol 106:893–904. https://doi.org/10.1007/s00392-017-1135-9
Coiro S, Girerd N, Rossignol P, Bauersachs J, Pitt B, Fay R, Ambrosio G, Solomon SD, Dickstein K, Zannad F (2017) Association of digitalis treatment with outcomes following myocardial infarction in patients with heart failure or evidence of left ventricular dysfunction: an analysis from the High-Risk Myocardial Infarction Database Initiative. Clin Res Cardiol. https://doi.org/10.1007/s00392-017-1116-z (Epub ahead of print)
Fröhlich H, Torres L, Täger T, Schellberg D, Corletto A, Kazmi S, Goode K, Grundtvig M, Hole T, Katus HA, Cleland JGF, Atar D, Clark AL, Agewall S, Frankenstein L (2017) Bisoprolol compared with carvedilol and metoprolol succinate in the treatment of patients with chronic heart failure. Clin Res Cardiol. https://doi.org/10.1007/s00392-017-1115-0 (Epub ahead of print)
Oldenburg O, Spießhöfer J, Fox H, Bitter T, Horstkotte D (2015) Cheyne–Stokes respiration in heart failure: friend or foe? Hemodynamic effects of hyperventilation in heart failure patients and healthy volunteers. Clin Res Cardiol 104:328–333. https://doi.org/10.1007/s00392-014-0784-1
Ishihara S, Gayat E, Sato N, Arrigo M, Laribi S, Legrand M, Placido R, Manivet P, Cohen-Solal A, Abraham WT, Jessup M, Mebazaa A (2016) Similar hemodynamic decongestion with vasodilators and inotropes: systematic review, meta-analysis, and meta-regression of 35 studies on acute heart failure. Clin Res Cardiol 105:971–980. https://doi.org/10.1007/s00392-016-1009-6
Ganz W, Donoso R, Marcus HS, Forrester JS, Swan HJ (1971) A new technique for measurement of cardiac output by thermodilution in man. Am J Cardiol 27:392–396
Swan HJ, Ganz W, Forrester J, Marcus H, Diamond G, Chonette D (1970) Catheterization of the heart in man with use of a flow-directed balloon-tipped catheter. N Engl J Med 283:447–451
Steinman TI, Becker BN, Frost AE, Olthoff KM, Smart FW, Suki WN (2001) Guidelines for the referral and management of patients eligible for solid organ transplantation. Transplantation 71:1189–1204
Fox H, Seeger FH, Schmitt J, Potente M, Dzemali O, Fichtlscherer S (2012) Veno-arterial ECMO as bridge to recovery. Cardiogenic shock and suspected myocarditis in a 37-year-old patient. Med Klin Intensivmed Notfmed 107:206–212
Fox H, Farr M, Horstkotte D, Flottmann C (2017) Fulminant myocarditis managed by extracorporeal life support (Impella® CP): a rare case. Case Rep Cardiol. https://doi.org/10.1155/2017/9231959
Hadian M, Pinsky MR (2006) Evidence-based review of the use of the pulmonary artery catheter: impact data and complications. Crit Care Suppl 3:S8
Rajaram SS, Desai NK, Kalra A, Gajera M, Cavanaugh SK, Brampton W, Young D, Harvey S, Rowan K (2013) Pulmonary artery catheters for adult patients in intensive care. Cochrane Database Syst Rev. (2):CD003408
Shah MR, Hasselblad V, Stevenson LW, Binanay C, O’Connor CM, Sopko G, Califf RM (2005) Impact of the pulmonary artery catheter in critically ill patients: meta-analysis of randomized clinical trials. JAMA 294:1664–1670
Vincent JL, Rhodes A, Perel A, Martin GS, Della Rocca G, Vallet B, Pinsky MR, Hofer CK, Teboul JL, de Boode WP, Scolletta S, Vieillard-Baron A, De Backer D, Walley KR, Maggiorini M, Singer M (2011) Clinical review: Update on hemodynamic monitoring—a consensus of 16. Crit Care 15:229. https://doi.org/10.1186/cc10291
Saugel B, Bendjelid K, Critchley LA, Rex S, Scheeren TW (2017) Journal of Clinical Monitoring and Computing 2016 end of year summary: cardiovascular and hemodynamic monitoring. J Clin Monit Comput 31:5–17. https://doi.org/10.1007/s10877-017-9976-3
Renner J, Grünewald M, Bein B (2016) Monitoring high-risk patients: minimally invasive and non-invasive possibilities. Best Pract Res Clin Anaesthesiol 30:201–216. https://doi.org/10.1016/j.bpa.2016.04.006
Sangkum L, Liu GL, Yu L, Yan H, Kaye AD, Liu H (2016) Minimally invasive or noninvasive cardiac output measurement: an update. J Anesth 30:461–480. https://doi.org/10.1007/s00540-016-2154-9
Saugel B, Cecconi M, Wagner JY, Reuter DA (2015) Noninvasive continuous cardiac output monitoring in perioperative and intensive care medicine. Br J Anaesth 114:562–575. https://doi.org/10.1093/bja/aeu447
Molhoek GP, Wesseling KH, Settels JJ, van Vollenhoven E, Weeda HW, de Wit B, Arntzenius AC (1984) Evaluation of the Penàz servo-plethysmo-manometer for the continuous, non-invasive measurement of finger blood pressure. Basic Res Cardiol 79:598–609
CNSystems Medizintechnik AG (2017) CNAP Monitor 500 HD. http://www.cnsystems.com/products. Accessed 10 Aug 2017
Jeleazcov C, Krajinovic L, Munster T, Birkholz T, Fried R, Schuttler J, Fechner J (2010) Precision and accuracy of a new device (CNAPTM) for continuous non-invasive arterial pressure monitoring: assessment during general anaesthesia. Br J Anaesth 105:264–272
Ilies C, Bauer M, Berg P, Rosenberg J, Hedderich J, Bein B, Hinz J, Hanss R (2012) Investigation of the agreement of a continuous non-invasive arterial pressure device in comparison with invasive radial artery measurement. Br J Anaesth 108:202–210
Marik PE (2013) Noninvasive cardiac output monitors: a state-of the-art review. J Cardiothorac Vasc Anesth 27:121–134
Monnet X, Teboul JL (2015) Minimally invasive monitoring. Crit Care Clin 31:25–42
Montenij LJ, Sonneveld JP, Nierich AP, Buhre WF, De Waal EE (2016) Accuracy, precision, and trending ability of uncalibrated arterial pressure waveform analysis of cardiac output in patients with impaired left ventricular function: a prospective, observational study. J Cardiothorac Vasc Anesth 30:115–121. https://doi.org/10.1053/j.jvca.2015.07.022
Du Bois D, Du Bois EF (1989) A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition 5:303–311
Bland JM, Altman DG (1999) Measuring agreement in method comparison studies. Stat Methods Med Res 8:135–160
Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1:307–310
Grouven U, Bender R, Ziegler A, Lange S (2007) Vergleich von Messmethoden. Dtsch med Wochenschr 132:e69–e73
Critchley LA, Critchley JA (1999) A meta-analysis of studies using bias and precision statistics to compare cardiac output measurement techniques. J Clin Monit Comput 15:85–91
Wagner JY, Körner A, Schulte-Uentrop L, Kubik M, Reichenspurner H, Kluge S, Reuter DA, Saugel B (2017) A comparison of volume clamp method-based continuous noninvasive cardiac output (CNCO) measurement versus intermittent pulmonary artery thermodilution in postoperative cardiothoracic surgery patients. J Clin Monit Comput. https://doi.org/10.1007/s10877-017-0027-x
Wagner JY, Grond J, Fortin J, Negulescu I, Schöfthaler M, Saugel B (2016) Continuous noninvasive cardiac output determination using the CNAP system: evaluation of a cardiac output algorithm for the analysis of volume clamp method-derived pulse contour. J Clin Monit Comput 30:487–493. https://doi.org/10.1007/s10877-015-9744-1
Bogert LW, Wesseling KH, Schraa O, Van Lieshout EJ, De Mol BA, Van Goudoever J, Westerhof BE, Van Lieshout JJ (2010) Pulse contour cardiac output derived from non-invasive arterial pressure in cardiovascular disease. Anaesthesia 65:1119–1125
Fischer MO, Avram R, Cârjaliu I, Massetti M, Gérard JL, Hanouz JL, Fellahi JL (2012) Non-invasive continuous arterial pressure and cardiac index monitoring with Nexfin after cardiac surgery. Br J Anaesth 109:514–521. https://doi.org/10.1093/bja/aes215
Ameloot K, Van De Vijver K, Broch O, Van Regenmortel N, De Laet I, Schoonheydt K, Dits H, Bein B, Malbrain ML (2013) Nexfin noninvasive continuous hemodynamic monitoring: validation against continuous pulse contour and intermittent transpulmonary thermodilution derived cardiac output in critically ill patients. Sci World J 2013:11. https://doi.org/10.1155/2013/519080519080.
Vetrugno L, Costa MG, Spagnesi L, Pompei L, Chiarandini P, Gimigliano I, Della Rocca G (2011) Uncalibrated arterial pulse cardiac output measurements in patients with moderately abnormal left ventricular function. J Cardiothorac Vasc Anesth 25:53–58. https://doi.org/10.1053/j.jvca.2010.07.001
Smetkin AA, Hussain A, Kuzkov VV, Bjertnæs LJ, Kirov MY (2014) Validation of cardiac output monitoring based on uncalibrated pulse contour analysis vs transpulmonary thermodilution during off-pump coronary artery bypass grafting. Br J Anaesth 112:1024–1031. https://doi.org/10.1093/bja/aet489
Ganter MT, Alhashemi JA, Al-Shabasy AM, Schmid UM, Schott P, Shalabi SA, Badri AM, Hartnack S, Hofer CK (2016) Continuous cardiac output measurement by un-calibrated pulse wave analysis and pulmonary artery catheter in patients with septic shock. J Clin Monit Comput 30:13–22. https://doi.org/10.1007/s10877-015-9672-0
Broch O, Renner J, Höcker J, Gruenewald M, Meybohm P, Schöttler J, Steinfath M, Bein B (2011) Uncalibrated pulse power analysis fails to reliably measure cardiac output in patients undergoing coronary artery bypass surgery. Crit Care 15:R76. https://doi.org/10.1186/cc10065
Monnet X, Anguel N, Naudin B, Jabot J, Richard C, Teboul JL (2010) Arterial pressure-based cardiac output in septic patients: different accuracy of pulse contour and uncalibrated pressure waveform devices. Crit Care 14:R109. https://doi.org/10.1186/cc9058
Biancofiore G, Critchley LA, Lee A, Bindi L, Bisà M, Esposito M, Meacci L, Mozzo R, DeSimone P, Urbani L, Filipponi F (2009) Evaluation of an uncalibrated arterial pulse contour cardiac output monitoring system in cirrhotic patients undergoing liver surgery. Br J Anaesth 102:47–54. https://doi.org/10.1093/bja/aen343
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Roth, S., Fox, H., Fuchs, U. et al. Noninvasive pulse contour analysis for determination of cardiac output in patients with chronic heart failure. Clin Res Cardiol 107, 395–404 (2018). https://doi.org/10.1007/s00392-017-1198-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-017-1198-7