Abstract
Background
There are many advantages of a SMOF emulsion (SMOF-lipid), such as liver-protective properties and anti-inflammatory effects. The objective of this study was to compare the clinical outcomes of SMOF-lipid with medium-chain triglycerides (MCT) /long-chain triglycerides (LCT) in infants after intestinal surgery.
Methods
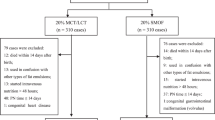
This was a prospective, randomized study. Neonates receiving intravenous nutrient solution, including lipid emulsion after gastrointestinal surgery, were included in this study. The patients were randomly assigned to the SMOF-lipid or MCT/LCT groups. Infants who received intravenous lipid emulsion continuously for > 2 weeks were considered to have completed the study. Differences in weight gain, nutrition indices, alanine transaminase (ALT), aspartate transaminase (AST), and direct bilirubin (DB), and inflammation cytokine markers (interleukin [IL]-6 and tumor necrosis factor [TNF]-α) were measured.
Results
The final sample included 160 infants. One hundred fourteen infants received intravenous SMOF-lipid (74) or MCT/LCT (86) > 2 weeks and 46 infants received intravenous SMOF-lipid (22) or MCT/LCT (24) > 4 weeks. There were no significant differences in weight gain, nutrition indices, inflammation cytokine markers, and sepsis between the groups at the end of 2 and 4 weeks; however, in the SMOF group, the ALT, AST, and DB levels were significantly lower than the MCT/LCT group at the end of 4 weeks.
Conclusion
The mixture and balanced emulsion of SMOF-lipid was well-tolerated in infants who have undergone gastrointestinal surgery, and liver-protective properties were demonstrated following long-term venous nutrition, especially > 4 weeks.






Similar content being viewed by others
References
Lee WS, Sokol RJ (2015) Intestinal microbiota, lipids, and the pathogenesis of intestinal failure—associated liver disease. J Pediatr 167:519–526
Squires RH, Balint J, Horslen S et al (2014) Race affects outcome among infants with intestinal failure. J Pediatr Gastroenterol Nutr 59:537–543
Goulet O, Joly F, Corriol O, Colomb-Jung V (2009) Some new insights in intestinal failure-associated liver disease. Curr Opin Organ Transpl 14:256–261
Grimm H (2005) A balanced lipid emulsion—a new concept in parenteral nutrition. Clin Nutr Suppl 1:25–30
Waitzberg DL, Torrinhas RS, Jacintho TM (2006) New parenteral lipid emulsions for clinical use. JPEN J Parenter Enteral Nutr 30:351–367
Waitzberg DL, Torrinhas RS (2009) Fish oil lipid emulsions and immune response: what clinicians need to know. Nutr Clin Pract 24:487–499
Vlaardingerbroek H, Ng K, Stoll B et al (2014) New generation lipid emulsions prevent PNALD in chronic parenteral nutrition fed preterm pigs. J Lipid Res 55:466–477
Schlotzer E, Kanning U (2004) Elimination and tolerance of a new parenteral lipid emulsion (SMOF)—a double-blind cross-over study in healthy male volunteers. Ann Nutr Metab 48:263–268. 15
Klek S, Chambrier C, Singer P et al (2013) Four-week parenteral nutrition using a third generation lipid emulsion (SMOFlipid)-a double-blind, randomised, multicentre study in adults. Clin Nutr 32:224–231
Calder PC (2009) Hot topics in parenteral nutrition. Rationale for using new lipid emulsions in parenteral nutrition and a review of the trials performed in adults. Proc Nutr Soc 68:252–260
Zugasti Murillo A, Petrina Jáuregui E, Elizondo Armendáriz J (2015) Parenteral nutrition-associated liver disease and lipid emulsions. Endocrinol Nutr 62:285–289
Calder PC, Jensen GL, Koletzko BV et al (2010) Lipid emulsions in parenteral nutrition of intensive care patients: current thinking and future directions. Intensive Care Med 36:735–749
Demmelmair H, Koletzko B (2015) Importance of fatty acids in the perinatal period. World Rev Nutr Diet 112:31–47
Wanten GJ, Calder PC (2007) Immune modulation by parenteral lipid emulsions. Am J Clin Nutr 85:1171–1184
Kumpf VJ (2006) Parenteral nutrition-associated liver disease in adult and pediatric patients. Nutr Clin Pract 21:279–290
Carpentier YA, Deckelbaum RJ (2015) In vivo handling and metabolism of lipid emulsions. World Rev Nutr Diet 112:57–62
Siqueira J, Smiley D, Newton C et al (2011) Substitution of standard soybean oil with olive oil-based lipid emulsion in parenteral nutrition: comparison of vascular, metabolic, and inflammatory effects. J Clin Endocrinol Metab 96:3207–3216
Carter BA, Karpen SJ (2007) Intestinal failure-associated liver disease: management and treatment strategies past, present, and future. Semin Liver Dis 27:251–258
Cavicchi M, Beau P, Crenn P et al (2000) Prevalence of liver disease and contributing factors in patients receiving home parenteral nutrition for permanent intestinal failure. Ann Intern Med 132:525–532
Calkins KL, DeBarber A, Steiner RD et al. Intravenous fish oil and pediatric intestinal failure-associated liver sisease: changes in plasma phytosterols, cytokines, and bile acids and erythrocyte fatty acids. JPEN J Parenter Enteral Nutr. 2017. https://doi.org/10.1177/0148607117709196. (Epub ahead of print)
Fuchs J, Fallon EM, Gura KM, Puder M (2011) Use of an omega-3 fatty acid-based emulsion in the treatment of parenteral nutrition-induced cholestasis in patients with microvillous inclusion disease. J Pediatr Surg 46:2376–2382
Meisel JA, Le HD, de Meijer VE et al (2011) Comparison of 5 intravenous lipid emulsions and their effects on hepatic steatosis in a murine model. J Pediatr Surg 46:666–673
Dai YJ, Sun LL, Li MY et al (2016) Comparison of formulas based on lipid emulsions of olive oil, soybean oil, or several oils for parenteral nutrition: a systematic review and meta-analysis. Adv Nutr 7:279–286
Hermans D, Talbotec C, Lacaille F et al (2007) Early central catheter infections may contribute to hepatic fibrosis in children receiving long-term parenteral nutrition. J Pediatr Gastroenterol Nutr 44:459–463
Goulet O, Olieman J, Ksiazyk J et al (2013) Neonatal short bowel syndrome as a model of intestinal failure: physiological background for enteral feeding. Clin Nutr 32:162–171
Goulet O, Antebi H, Wolf C et al (2010) A new intravenous fat emulsion containing soybean oil, medium-chain triglycerides, olive oil, and fish oil: a single-center, double-blind randomized study on efficacy and safety in pediatric patients receiving home parenteral nutrition. J Parenter Enteral Nutr 34:485–495
Gura KM, Lee S, Valim C et al (2008) Safety and efficacy of a fish-oil-based fat emulsion in the treatment of parenteral nutrition associated liver disease. Pediatrics 121:e678–e686
Diamond IR, Sterescu A, Pencharz PB et al (2008) The rationale for the use of parenteral omega-3 lipids in children with short bowel syndrome and liver disease. Pediatr Surg Int 24:773–778
Zhao Y, Wu Y, Pei J,et al (2015) Safety and efficacy of parenteral fish oil-containing lipid emulsions in premature neonates. J Pediatr Gastroenterol Nutr 60:708–716
Angsten G, Finkel Y, Lucas S et al. Improved outcome in neonatal short bowel syndrome using parenteral fish oil in combination with ω-6/9 lipid emulsions. 2012;36:587–595
Deshpande G, Simmer K, Deshmukh M et al (2014) Fish oil (SMOFlipid) and olive oil lipid (clinoleic) in very preterm neonates. J Pediatr Gastroenterol Nutr 58:177–182
Schade I, Röhm KD, Schellhaass A et al (2008) Inflammatory response in patients requiring parenteral nutrition: comparison of a new fish oil-containing emulsion (SMOF) versus an olive/soybean oil-based formula. Crit Care 12:144
Calder PC (2015) Functional roles of fatty acids and their effects on human health. JPEN J Parenter Enteral Nutr 39(1 Suppl):18S–32S
Calder PC (2015) Marine omega-3 fatty acids and inflammatory processes: effects, mechanisms and clinical relevance. Biochim Biophys Acta 1851:469–484
Nanhuck RM, Doublet A, Yaqoob P (2009) Effects of lipid emulsions on lipid body formation and eicosanoid production by human peripheral blood mononuclear and polymorphonuclear cells. Clin Nutr 28(5):556–564
Simopoulos AP (2006) Evolutionary aspects of diet, the omega-6/omega-3 ratio and genetic variation: nutritional implications for chronic disease. Biomed Pharmacother 60(9):502e7
Grimm H, Mertes N, Goeters C et al (2006) Improved fatty acid and leukotriene pattern with a novel lipid emulsion in surgical patients. Eur J Nutr 45:55–60
Vlaardingerbroek H, van Goudoever JB (2015) Intravenous lipids in preterm infants: impact on laboratory and clinical outcomes and long-term consequences. World Rev Nutr Diet 112:71–80
Ghazale H, Ramadan N, Mantash S et al (2018) Docosahexaenoic acid (DHA) enhances the therapeutic potential of neonatal neural stem cell transplantation post-Traumatic brain injury. Behav Brain Res 340:1–13
Beken S, Dilli D, Fettah ND et al (2014) The influence of fish-oil lipid emulsions on retinopathy of prematurity in very low birth weight infants: a randomized controlled trial. Early Hum Dev 90:27–31
Nehra D, Fallon EM, Potemkin AK et al (2014) A comparison of 2 intravenous lipid emulsions: interim analysis of a randomized controlled trial. JPEN J Parenter Enteral Nutr 38:693–701
Kidokoro H, Neil JJ, Inder TE (2013) New MR imaging assessment tool to define brain abnormalities in very preterm infants at term. AJNR Am J Neuroradiol 34:2208–2214
Soden JS, Lovell MA, Brown K et al (2010) Failure of resolution of portal fibrosis duringomega-3 fatty acid lipid emulsion therapy in two patients with irreversible intestinalfailure. J Pediatr 156:327–331
Acknowledgements
This study was supported by the National Natural Science Foundation of China (81100318), the Jiangsu Provincial key research and development program (BE2017609), Jiangsu youth medical talent project (QNRC2016081), and Children’s Hospital of Nanjing Medical University youth medical talent project (ETYYQM2014009), Nanjing Science and Technology Project (201823015).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Jiang, W., Chen, G., Zhang, J. et al. The effects of two mixed intravenous lipid emulsions on clinical outcomes in infants after gastrointestinal surgery: a prospective, randomized study. Pediatr Surg Int 35, 347–355 (2019). https://doi.org/10.1007/s00383-018-4422-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-018-4422-2