Abstract
Aim of the study
Complex tracheo-oesophageal fistulae (TOF) are rare congenital or acquired conditions in children. We discuss here a multidisciplinary (MDT) approach adopted over the past 5 years.
Methods
We retrospectively collected data on all patients with recurrent or acquired TOF managed at a single institution. All cases were investigated with neck and thorax CT scan. Other investigations included flexible bronchoscopy and bronchogram (B&B), microlaryngobronchoscopy (MLB) and oesophagoscopy. All cases were subsequently discussed in an MDT meeting on an emergent basis if necessary.
Main results
14 patients were referred during this study period of which half had a congenital aetiology and the other half were acquired. The latter included button battery ingestions (5/7) and iatrogenic injuries during oesophageal atresia (OA) repair. Surgical repair was performed on cardiac bypass in 3/7 cases of recurrent congenital fistulae and all cases of acquired fistulae. Post-operatively, 9/14 (64%) patients suffered complications including anastomotic leak (1), bilateral vocal cord paresis (1), further recurrence (1), and mortality (1). Ten patients continue to receive surgical input encompassing tracheal/oesophageal stents and dilatations.
Conclusions
MDT approach to complex cases is becoming increasingly common across all specialties and is important in making decisions in these difficult cases. The benefits include shared experience of rare cases and full access to multidisciplinary expertise.
Similar content being viewed by others
Introduction
Complex tracheo-oesophageal fistulae (TOF) are rare conditions in children [1]. Most often these occur after repair of congenital oesophageal atresia (OA) with a distal TOF (C type). Primary repair has a re-fistulisation rate of 3–5% [1, 2]. Complex TOFs can also be a result of oesophageal injury by ingestion of caustic fluids or button batteries [3, 4].
The surgical repair of complex recurrent TOF or acquired lesions is challenging for a number of reasons. First, the diagnosis itself can be difficult and often requires oesophageal contrast studies and endoscopies to confirm and intubate the fistula. The literature suggests that a prone oesophagogram whilst withdrawing an NG tube is the most sensitive investigation with the fewest false negatives [5]. The most sensitive test in our experience has been to perform bronchography with bronchoscopic (B&B) probing of the fistula pit.
The quality of tissues may present a surgical challenge, particularly in acquired injuries where the defects tend to be larger and the tissues friable with areas of ischaemia or even frank necrosis. In recurrent TOF the scarring due to previous surgery also makes the access and safe mobilisation of the trachea and oesophagus more difficult, increasing the risk of intra- and post-operative complications.
Different approaches for managing these fistulae have been described: endoscopic use of glue for small defects [6], standard thoracotomy with a variety of adjuncts such as interposition flaps [7] (pericardium, pleura), early abandonment of the oesophagus in preference of tracheal preservation and median sternotomy with or without the use of cardiopulmonary bypass (CPB) for the more complex cases [8, 9].
Following a recent increase in tertiary referrals of complex TOF cases to our institution, a multidisciplinary (MDT) approach has been adopted so as to treat every patient on an individual basis. In this paper, we discuss our MDT approach based on the case series of complex TOFs treated in our institution over the past 5 years.
Methods
We retrospectively collected data on all patients with recurrent or acquired TOF managed at Great Ormond Street Hospital from January 2013 till July 2018. All patients were tertiary referrals from other UK or international surgical centres. All cases were investigated with contrast CT scan of the neck and thorax. Other investigations included B&B, MLB and oesophagoscopy.
All cases were subsequently discussed in our MDT meeting (Table 1). We followed a 3 “Ds” approach—Diagnosis, Discussion and Decision-making. Those present at our MDT include a cardiothoracic surgeon, diagnostic radiologist, ENT surgeon, general paediatric surgeon, intensive care physician, interventional radiologist and radiographer, respiratory physician and specialist nurses. We have specifically incorporated the experience of our specialist nurses into this MDT setting to create a complex aero-digestive team. Decisions on the approach to be used in each case were based on several factors. Size, position of defect in the trachea and aetiology were the most important factors when considering whether CPB was required for repair or whether an endotracheal tube could be safely placed distal to the TOF allowing conventional ventilation. Size of defect also determined whether complete circumferential control of the trachea may be needed for division and repair of the trachea—necessitating a sternotomy approach. Surgical history of repeated thoracotomy was also a relative indication for sternotomy.
Fisher’s test and chi-squared tests were used for statistical analysis with P < 0.05 considered statistically significant.
Results
14 patients were referred during this study period of which half had a congenital aetiology (C-TOF) and the other half acquired (A-TOF). Two patients were referred from outside the UK, with the remaining referred from other UK tertiary paediatric surgical centres. Table 2 summarises the patient demographics, medical history, reasons for referral to our institution, the nature of surgery undertaken at our centre and their outcomes. The aetiology for the majority of acquired fistulae was button battery ingestion with two iatrogenic cases from repair of oesophageal atresia.
Figures 1, 2, 3 and 4 illustrate bronchograms and endoscopic images of patients in our series with complex fistulae.
Surgical approach
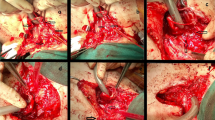
Figure 5 illustrates the surgical approach for each of the cases. Surgical repair was performed in all cases with CPB used in 3/7 (43%) cases of congenital recurrent fistulae and all cases (100%) of acquired fistulae. The median bypass time was 118 min (91–180 min).
Oesophageal replacement
Major emphasis is made to preserve tracheal tissue at all costs for obvious reasons. We have also tried to adopt a conservative approach for the oesophagus when possible. In 4/14 (30%) of cases, it was sacrificed only after a second exploration. Figure 6 illustrates retention of the native oesophagus in our cohort of patients. When removed, patients received an oesophagostomy with a plan for future oesophageal replacement. Our preferred method for this has been laparoscopic-assisted gastric transposition.
Complications and follow-up
Surgery for complex TOFs is challenging and despite MDT discussion and careful surgical planning, minor or major complications in 8/14 (60%) patients exist (Fig. 7).
There was one death in a patient who initially underwent a thoracotomy and repair of OA/TOF at one referring institution. The infant developed a recurrent fistula at 2 months of age which was operated on again through a thoracotomy. Unfortunately, the fistula recurred again and the patient was transferred intubated and ventilated from paediatric intensive care to our institution. He underwent a repair of this fistula under CPB with an autologous pericardial tracheoplasty and direct oesophageal repair. This recurred again and further surgery was performed under CBP including an oesophagostomy and gastrostomy. One week later he suffered from an acute deterioration with left-sided tension pneumothorax. He was stabilised from this but continued to suffer from an ongoing air leak. This was explored through a sternotomy on extra-corporeal membrane oxygenation (ECMO) and after discovering tracheal necrosis with active infection the MDT decision was to not proceed any further.
10 patients continue to receive surgical input at our institution with 3 patients now cared for at their original referring centre. Figure 8 summarises the treatments used for these patients as part of their ongoing management at our institution.
Aerodigestive outcomes
The majority of all our patients are currently orally fed. 2/7 (30%) patients in the acquired group are having also need additional feeds via a gastrostomy or jejunostomy. From an airway perspective, none of our patients are currently receiving supplemental oxygen. 1/7 (14%) patient in the acquired group needed a tracheostomy for transient bilateral vocal cord palsy which has now been decannulated.
Discussion
Complex cases of acquired and recurrent TOF are rare in children and as such require careful consideration of the best and safest approach to their management. At our institution, we have developed an MDT approach to these cases with individualised management directed towards their complex needs. Our patient cohort can essentially be divided into two cohorts: (1) congenital recurrent fistulae and (2) acquired cases largely from button battery ingestion. Our experience with the latter group has been more challenging with the outcomes similarly reflecting the complexity of the condition.
The incidence of recurrent congenital tracheo-oesophageal fistula has been estimated to be 3–14% [1]. The diagnosis of a recurrent fistula can be difficult and often requires a combination of radiological and endoscopic studies. The prone tube oesophagogram is a reasonable first test. If, however, this test is negative and high index of clinical suspicion exists, we would proceed to a B&B with probing of the fistula pit. The bronchoscopy can be flexible or rigid but should be performed with high-quality fluoroscopy in lateral projection and preferably biplane.
Whereas endoscopic management alone of these has been described [7], our preference has been to perform surgical repair because of the high risk of recurrence after conservative management. In a systematic review of 165 patients across 44 studies [5], the success rate of surgery was found to be 93.5% compared with 84% for endoscopic treatment. In our study, patients had either failed endoscopic management prior to their referral to our institution or they were too large to be suitable for this approach. For this reason, all patients in our study underwent surgical repair.
Ingested button batteries can cause severe and rapid injury to mediastinal structures. This problem has recently received a lot of media attention through several recent high-profile cases [10]. In the US, the National Poison Data System estimates an incidence of 6.3–15.1 button battery ingestions per million of the population annually [11]. In the UK, the Child Accident Prevention Trust and British and Irish Portable Battery Association have both launched recent campaigns highlighting the potential dangers to families. In our cohort of acquired fistulae, 5/7 (71%) of cases were due to button batteries and all these cases were managed surgically. Conservative management of such fistulae has also been reported [11]; however, we believe that this is not effective largely due to the friable nature of the tissue and the presence of ischaemia or necrosis. As a specialist referral centre, we also tend to see patients on the severe end of the spectrum which tends to skew our preference towards surgical management.
Several operative approaches have been described in the literature when approaching these complex fistulae [7,8,9]. The decision of which approach is most suitable is made within our MDT meetings with sufficient detailed imaging allowing a full understanding of the anatomy pre-operatively. We, therefore, perform a combination of cross-sectional imaging (CT thorax) and contrast studies or endoscopy of airway and oesophagus. The key factors to consider are the size and position of the fistula, quality of tissue, type of injury, previous surgery and co-morbidities.
The size and position of the fistula, in particular, are pertinent in determining whether ventilation will be possible using a distally placed endotracheal tube. The more distal the fistula the harder it becomes to reliably ventilate the patient. Tissue quality is also a problem after surgery for recurrent cases or battery injuries. It must also be remembered the fistulae will probably be made larger following dissection or debridement of necrotic tissue. Although it may be possible to safely ventilate these patients pre-operatively, this may change significantly following dissection of the defect.
In some patients that had a congenital aetiology with at least two previous recurrences, direct repair of the trachea or a more complex tracheoplasty required maximal control of ventilation and oxygenation. In those cases, we have, therefore, opted to perform the repair on CPB through a midline sternotomy as opposed to conventional thoracotomy [12]. Provenzano et al. [13] report utilising CPB in patients with distal TOF for which they favour a slide tracheoplasty repair, but do not describe in detail their decision-making process for this. Tibballs et al. have also demonstrated the need for CPB in the case of a huge relatively proximal TOF from a button battery injury in which adequate oxygenation could not be adequately achieved with conventional ventilation [14].
The complexity of the patients that have been referred to our institution is clearly evident from this report with our outcomes reflective of the severity of the conditions. As an institution, we often receive a select population of patients which have already previously failed conservative or surgical management.
There was one death (7%) in the study group in a patient with a persistent, recurrent congenital TOF who died from tracheal necrosis following four attempts at repair. Wang et al. in their series of 35 patients with a recurrent fistula report a mortality rate of 8.6% [1]. In their series, the patients died from chest infections/sepsis following surgery. In our patient, repeated surgery on the trachea led to eventual necrosis and despite a trial of ECMO the patient did not survive. One further patient in the congenital group referred with a persistent TOF developed a recurrence after surgery. This patient required surgery on CPB on two further occasions and has required a biodegradable tracheal stent with ongoing tracheal and oesophageal dilatations.
In the acquired group, 3/7 (43%) patients have had further recurrences. The high incidence of recurrence and leaks in our series is a result of several factors. First, the complexity of the defects, especially those at the carina makes successful primary repair more difficult. Second, large defects on the oesophageal end of the fistula are often repaired directly and are prone to leakage and subsequent fistulation due to the friable nature of the tissue.
Other complications encountered in our series were tracheal and oesophageal strictures that were treated regularly with dilatations. The acquired group, in particular, required significantly more oesophageal dilatations (86%) compared with the congenital group (14%). A similar experience has recently been reported in a series of patients from the Netherlands [15]. As already discussed, this is largely due to the damage caused to the oesophagus through pressure necrosis, chemical alkaline damage and generation of an electrical current [15].
In conclusion, the MDT approach to complex cases is becoming increasingly common across all specialties and is important in making decisions in these difficult cases. The benefits of such an MDT approach are well recognised at other leading centres dealing with a similar complex case mix [16]. When faced with such complex patients, taking an MDT approach enables holistic care to be delivered to the patient. Furthermore, each member of the team can draw on their experience and reciprocally the proficiency of the group is enhanced with each case faced collectively. We advocate early referral of these complex cases to centres where such expertise is available to offer the full range of treatment to patients.
References
Wang J, Zhang M, Pan W, Wu W et al (2017) Management of recurrent tracheoesophageal fistula after esophageal atresia and follow-up. Dis Esophagus 30(9):1–8
Sugiyama A, Urushihara N, Fukumoto K, Fukuzawa H et al (2013) Combined free autologous auricular cartilage and fascia lata graft repair for a recurrent tracheoesophageal fistula. Pediatr Surg Int 29(5):519–523
Rosskopfova P, Perentes JY, Schäfer M, Krueger T et al (2017) Repair of challenging non-malignant tracheo- or broncho-oesophageal fistulas by extrathoracic muscle flaps. Eur J Cardiothorac Surg 51(5):844–851
Collins NF, Ellard L, Licari E, Beasley E et al (2014) Veno-venous extracorporeal membrane oxygenation and apnoeic oxygenation for tracheo-oesophageal fistula repair in a previously pneumonectomised patient. Anaesth Intensive Care 42(6):789–792
Aworanti O, Awadalla S (2014) Management of recurrent tracheoesophageal fistulas: a systematic review. Eur J Pediatr Surg 24(5):365–375
Muniappan A, Wain JC, Wright CD, Donahue DM et al. Surgical treatment of nonmalignant tracheoesophageal fistula: a thirty-five year experience. Ann Thorac Surg. 2013; (4):1141–6
Nazir Z, Khan MAM, Qamar J et al (2017) Recurrent and acquired tracheoesophageal fistulae (TEF)-Minimally invasive management. J Pediatr Surg 52(10):1688–1690
Pandey V, Gangopadhyay AN, Gupta DK, Sharma SP et al (2014) Novel technique of repair of large tracheo-esophageal fistula following battery ingestion in children: review of two cases. Pediatr Surg Int 30(5):537–539
Ettyreddy AR, Georg MW, Chi DH, Gaines BA et al (2015) Button battery injuries in the pediatric aerodigestive tract. Ear Nose Throat J 94(12):486–493
Great Ormond Street Hospital for Children (2016) UK’s top paediatric doctors warn of devastating impact of button batteries. [Online]. https://www.gosh.nhs.uk/news/latest-press-releases/2016-press-release-archive/uk-s-top-paediatric-doctors-warn-devastating-impact-button-batteries. Accessed 3 Sep 2018
Russell RT, Cohen M, Billmire DF (2013) Tracheoesophageal fistula following button battery ingestion: successful non-operative management. J Pediatr Surg 48(2):441–444
Smithers CJ, Hamilton TE, Manfredi MA, Rhein L (2017) Categorization and repair of recurrent and acquired tracheoesophageal fistulae occurring after esophageal atresia repair. J Pediatr Surg 52(3):424–430
Provenzano MJ, Rutter MJ, von Allmen D, Manning PB (2014) Slide tracheoplasty for the treatment of tracheoesophogeal fistulas. J Pediatr Surg 49(6):910–914
Tibballs J, Wall R, Koottayi SV, Stokes KB et al (2002) Tracheo-oesophageal fistula caused by electrolysis of a button battery impacted in the oesophagus. J Paediatr Child Health 38(2):201–203
Krom H, Visser M, Hulst JM, Wolters VM et al (2018) Serious complications after button battery ingestion in children. Eur J Pediatr 177(7):1063–1070
Lobeck I, Dupree P, Stoops M, de Alarcon A et al (2016) Interdisciplinary approach to esophageal replacement and major airway reconstruction. J Pediatr Surg 51(7):1106–1109
Aknowledgements
P.D.C. is supported by National Institute for Health Research (NIHR-RP-2014-04-046). All research at Great Ormond Street Hospital NHS Foundation Trust and UCL Great Ormond Street Institute of Child Health is made possible by the NIHR Great Ormond Street Hospital Biomedical Research Centre. The views expressed are those of the author (s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Thakkar, H.S., Hewitt, R., Cross, K. et al. The multi-disciplinary management of complex congenital and acquired tracheo-oesophageal fistulae. Pediatr Surg Int 35, 97–105 (2019). https://doi.org/10.1007/s00383-018-4380-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-018-4380-8