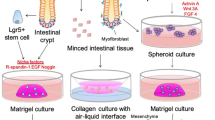
Abstract
Background
Adult intestinal organoids have been used to study ex vivo intestinal injury in adulthood. However, the neonatal intestinal epithelium has many unique features that are different from adult mature intestine. Establishing a neonatal ex vivo organoid model is essential to study the epithelial physiology in early postnatal development and to investigate derangements associated with disease processes during the neonatal period like necrotizing enterocolitis (NEC).
Methods
Fresh and frozen terminal ileum was harvested from mice pups on postnatal day 9. Crypts were isolated and organoids were cultured. Organoids were exposed to hypoxia and lipopolysaccharide (LPS) for 48 h to induce epithelial injury. Inflammatory cytokines and tight junction proteins were evaluated.
Results
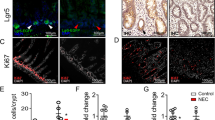
Robust intestinal organoids can be formed from both fresh and frozen intestinal tissue of neonatal mice pups. Hypoxia and LPS administration induced intestinal inflammation and disrupted tight junctions in these neonatal intestinal organoids.
Conclusions
We have established a novel method to grow organoids from neonatal intestine. We demonstrated that these organoids respond to the injury occurring during neonatal intestinal diseases such as NEC by increasing the organoid inflammation and by disrupting the organoid barrier function. Organoids provide an ex vivo platform to study intestinal physiology and pathology during the neonatal period.



Similar content being viewed by others
References
Walton KD, Freddo AM, Wang S, Gumucio DL (2016) Generation of intestinal surface: an absorbing tale. Development 143(13):2261–2272. https://doi.org/10.1242/dev.135400
Ley RE, Peterson DA, Gordon JI (2006) Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell 124(4):837–848. https://doi.org/10.1016/j.cell.2006.02.017
Cheng H, Leblond CP (1974) Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian Theory of the origin of the four epithelial cell types. Am J Anat 141(4):537–561. https://doi.org/10.1002/aja.1001410407
Sato T, Clevers H (2013) Growing self-organizing mini-guts from a single intestinal stem cell: mechanism and applications. Science 340(6137):1190–1194. https://doi.org/10.1126/science.1234852
Yui S, Nakamura T, Sato T, Nemoto Y, Mizutani T, Zheng X, Ichinose S, Nagaishi T, Okamoto R, Tsuchiya K, Clevers H, Watanabe M (2012) Functional engraftment of colon epithelium expanded in vitro from a single adult Lgr5(+) stem cell. Nat Med 18(4):618–623. https://doi.org/10.1038/nm.2695
Clevers H (2016) Modeling development and disease with organoids. Cell 165(7):1586–1597. https://doi.org/10.1016/j.cell.2016.05.082
Schweiger PJ, Jensen KB (2016) Modeling human disease using organotypic cultures. Curr Opin Cell Biol 43:22–29. https://doi.org/10.1016/j.ceb.2016.07.003
Dutta D, Heo I, Clevers H (2017) Disease modeling in stem cell-derived 3D organoid systems. Trends Mol Med 23(5):393–410. https://doi.org/10.1016/j.molmed.2017.02.007
Stenson WF (2013) Postnatal growth in the intestine. Curr Opin Gastroenterol 29(2):107–111. https://doi.org/10.1097/MOG.0b013e32835d9ec3
Herbst JJ, Sunshine P (1969) Postnatal development of the small intestine of the rat. Changes in mucosal morphology at weaning. Pediatr Res 3(1):27–33
Paran TS, Rolle U, Puri P (2006) Postnatal development of the mucosal plexus in the porcine small and large intestine. Pediatr Surg Int 22(12):997–1001. https://doi.org/10.1007/s00383-006-1786-5
Unthank JL, Lash JM, Bohlen HG (1990) Maturation of the rat intestinal microvasculature from juvenile to early adult life. Am J Physiol 259(2 Pt 1):G282–G289. https://doi.org/10.1152/ajpgi.1990.259.2.G282
Trahair JF (1989) Remodeling of the rat small intestinal mucosa during the suckling period. J Pediatr Gastroenterol Nutr 9(2):232–237
Dehmer JJ, Garrison AP, Speck KE, Dekaney CM, Van Landeghem L, Sun X, Henning SJ, Helmrath MA (2011) Expansion of intestinal epithelial stem cells during murine development. PLoS One 6(11):e27070. https://doi.org/10.1371/journal.pone.0027070
Rouwet EV, Heineman E, Buurman WA, ter Riet G, Ramsay G, Blanco CE (2002) Intestinal permeability and carrier-mediated monosaccharide absorption in preterm neonates during the early postnatal period. Pediatr Res 51(1):64–70. https://doi.org/10.1203/00006450-200201000-00012
Baumler AJ, Sperandio V (2016) Interactions between the microbiota and pathogenic bacteria in the gut. Nature 535(7610):85–93. https://doi.org/10.1038/nature18849
Cummins AG, Thompson FM (1997) Postnatal changes in mucosal immune response: a physiological perspective of breast feeding and weaning. Immunol Cell Biol 75(5):419–429. https://doi.org/10.1038/icb.1997.67
Lee C, Minich A, Li B, Miyake H, Seo S, Pierro A (2018) Influence of stress factors on intestinal epithelial injury and regeneration. Pediatr Surg Int 34(2):155–160. https://doi.org/10.1007/s00383-017-4183-3
Dekkers JF, Wiegerinck CL, de Jonge HR, Bronsveld I, Janssens HM, de Winter-de Groot KM, Brandsma AM, de Jong NW, Bijvelds MJ, Scholte BJ, Nieuwenhuis EE, van den Brink S, Clevers H, van der Ent CK, Middendorp S, Beekman JM (2013) A functional CFTR assay using primary cystic fibrosis intestinal organoids. Nat Med 19(7):939–945. https://doi.org/10.1038/nm.3201
Li B, Lee C, Filler T, Hock A, Wu RY, Li Q, Chen S, Koike Y, Ip W, Chi L, Zani-Ruttenstock E, Maattanen P, Gonska T, Delgado-Olguin P, Zani A, Sherman PM, Pierro A (2017) Inhibition of corticotropin-releasing hormone receptor 1 and activation of receptor 2 protect against colonic injury and promote epithelium repair. Sci Rep 7:46616. https://doi.org/10.1038/srep46616
Sodhi CP, Neal MD, Siggers R, Sho S, Ma C, Branca MF, Prindle T Jr, Russo AM, Afrazi A, Good M, Brower-Sinning R, Firek B, Morowitz MJ, Ozolek JA, Gittes GK, Billiar TR, Hackam DJ (2012) Intestinal epithelial Toll-like receptor 4 regulates goblet cell development and is required for necrotizing enterocolitis in mice. Gastroenterology 143(3):708–718 e705. https://doi.org/10.1053/j.gastro.2012.05.053
McElroy SJ, Castle SL, Bernard JK, Almohazey D, Hunter CJ, Bell BA, Al Alam D, Wang L, Ford HR, Frey MR (2014) The ErbB4 ligand neuregulin-4 protects against experimental necrotizing enterocolitis. Am J Pathol 184(10):2768–2778. https://doi.org/10.1016/j.ajpath.2014.06.015
Zani A, Cananzi M, Fascetti-Leon F, Lauriti G, Smith VV, Bollini S, Ghionzoli M, D’Arrigo A, Pozzobon M, Piccoli M, Hicks A, Wells J, Siow B, Sebire NJ, Bishop C, Leon A, Atala A, Lythgoe MF, Pierro A, Eaton S, De Coppi P (2014) Amniotic fluid stem cells improve survival and enhance repair of damaged intestine in necrotising enterocolitis via a COX-2 dependent mechanism. Gut 63(2):300–309. https://doi.org/10.1136/gutjnl-2012-303735
Ling X, Linglong P, Weixia D, Hong W (2016) Protective effects of bifidobacterium on intestinal barrier function in LPS-induced enterocyte barrier injury of Caco-2 monolayers and in a rat NEC model. PLoS One 11(8):e0161635. https://doi.org/10.1371/journal.pone.0161635
Khailova L, Dvorak K, Arganbright KM, Halpern MD, Kinouchi T, Yajima M, Dvorak B (2009) Bifidobacterium bifidum improves intestinal integrity in a rat model of necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol 297(5):G940–G949. https://doi.org/10.1152/ajpgi.00141.2009
Senger S, Ingano L, Freire R, Anselmo A, Zhu W, Sadreyev R, Walker WA, Fasano A (2018) Human fetal-derived enterospheres provide insights on intestinal development and a novel model to study necrotizing enterocolitis (NEC). Cell Mol Gastroenterol Hepatol 5(4):549–568. https://doi.org/10.1016/j.jcmgh.2018.01.014
Walsh AJ, Cook RS, Sanders ME, Arteaga CL, Skala MC (2016) Drug response in organoids generated from frozen primary tumor tissues. Sci Rep 6:18889. https://doi.org/10.1038/srep18889
Acknowledgements
This work is supported by a Canadian Institutes of Health Research (CIHR) Foundation Grant 353857. AP is the Robert M. Filler Chair of Surgery, The Hospital for Sick Children (HSC). BL is the recipient of HSC Restracomp Fellowship.
Author information
Authors and Affiliations
Contributions
BL, CL, and AP designed experiments; CL, MC, HM, DL, and RW performed experiments; BL, CL, and MC wrote the manuscript; AP provided advice and supervision; all the authors reviewed and revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interest.
Rights and permissions
About this article
Cite this article
Li, B., Lee, C., Cadete, M. et al. Neonatal intestinal organoids as an ex vivo approach to study early intestinal epithelial disorders. Pediatr Surg Int 35, 3–7 (2019). https://doi.org/10.1007/s00383-018-4369-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-018-4369-3