Abstract
Introduction
Hepatic dysfunction in patients reliant on total parenteral nutrition (TPN) may benefit from cycled TPN. A concern for neonatal hypoglycemia has limited the use of cycled TPN in neonates less than 1 week of age. We sought to determine both the safety and efficacy of cycled TPN in surgical neonates less than 1 week of age.
Methods
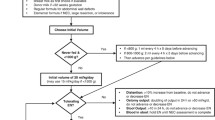
A retrospective chart review was conducted on surgical neonates placed on prophylactic and therapeutic cycled TPN from January 2013 to March 2016. Specific emphasis was placed on identifying incidence of direct hyperbilirubinemia and hypoglycemic episodes.
Results
Fourteen neonates were placed on cycled TPN; 8 were prophylactically cycled and 6 were therapeutically cycled. Median gestational age was 36 weeks (34, 37). Sixty-four percent (n = 9) had gastroschisis. There was no difference between the prophylactic and therapeutic groups in incidence of hyperbilirubinemia > 2 mg/dL (3 (37%) vs 5 (83%), p = 0.08) or the length of time to development of hyperbilirubinemia [24 days (4, 26) vs 27 days (25, 67), p = 0.17]. Time on cycling was similar though patients who were prophylactically cycled had a shorter overall time on TPN. Three (21%) infants had documented hypoglycemia, but only one infant became clinically symptomatic.
Conclusion
Prophylactic TPN cycling is a safe and efficacious nutritional management strategy in surgical neonates less than 1 week of age with low rates of hypoglycemia and a shorter total course of TPN; however, hepatic dysfunction did not appear to be improved compared to therapeutic cycling.

Similar content being viewed by others
Abbreviations
- GIR:
-
Glucose infusion rate
- TPN:
-
Total parenteral nutrition
References
Kubota A, Yonekura T, Hoki M et al (2000) Total parenteral nutrition-associated intrahepatic cholestasis in infants: 25 years’ experience. J Pediatr Surg 35:1049–1051. https://doi.org/10.1053/jpsu.2000.7769
Sondheimer JM, Asturias E, Cadnapaphornchai M (1998) Infection and cholestasis in neonates with intestinal resection and long-term parenteral nutrition. J Pediatr Gastroenterol Nutr 27:131–137
Wright K, Ernst KD, Gaylord MS et al (2003) Increased incidence of parenteral nutrition-associated cholestasis with aminosyn PF compared to trophamine. J Perinatol Off J Calif Perinat Assoc 23:444–450. https://doi.org/10.1038/sj.jp.7210965
Beath SV, Davies P, Papadopoulou A et al (1996) Parenteral nutrition-related cholestasis in postsurgical neonates: multivariate analysis of risk factors. J Pediatr Surg 31:604–606
Albers MJIJ, de Gast-Bakker D-AH, van Dam NAM et al (2002) Male sex predisposes the newborn surgical patient to parenteral nutrition-associated cholestasis and to sepsis. Arch Surg Chic Ill 1960 137:789–793
Slicker J, Vermilyea S (2009) Pediatric parenteral nutrition: putting the microscope on macronutrients and micronutrients. Nutr Clin Pract 24:481–486. https://doi.org/10.1177/0884533609339073
Nghiem-Rao TH, Cassidy LD, Polzin EM et al (2013) Risks and benefits of prophylactic cyclic parenteral nutrition in surgical neonates. Nutr Clin Pract Off Publ Am Soc Parenter Enter Nutr 28:745–752. https://doi.org/10.1177/0884533613502813
Austhof SI, DeChicco R, Cresci G et al (2017) Expediting transition to home parenteral nutrition with fast-track cycling. J Parenter Enter Nutr 41:446–454. https://doi.org/10.1177/0148607115595620
Meehan JJ, Georgeson KE (1997) Prevention of liver failure in parenteral nutrition-dependent children with short bowel syndrome. J Pediatr Surg 32:473–475
Collier S, Crough J, Hendricks K, Caballero B (1994) Use of cyclic parenteral nutrition in infants less than 6 months of age. Nutr Clin Pract Off Publ Am Soc Parenter Enter Nutr 9:65–68. https://doi.org/10.1177/011542659400900265
Jensen AR, Goldin AB, Koopmeiners JS et al (2009) The association of cyclic parenteral nutrition and decreased incidence of cholestatic liver disease in patients with gastroschisis. J Pediatr Surg 44:183–189. https://doi.org/10.1016/j.jpedsurg.2008.10.033
Stout SM, Cober MP (2010) Metabolic effects of cyclic parenteral nutrition infusion in adults and children. Nutr Clin Pract 25:277–281. https://doi.org/10.1177/0884533610368701
Takehara H, Hino M, Kameoka K, Komi N (1990) A new method of total parenteral nutrition for surgical neonates: it is possible that cyclic TPN prevents intrahepatic cholestasis. Tokushima J Exp Med 37:97–102
Funding
No funding was received to conduct this study.
Author information
Authors and Affiliations
Contributions
JS: Contributed to conception or design, drafted the manuscript, critically revised the manuscript, gave final approval, agrees to be accountable for all aspects of work ensuring integrity and accuracy. KW: Contributed to conception or design, contributed to acquisition of data, drafted the manuscript, critically revised the manuscript, gave final approval, agrees to be accountable for all aspects of work ensuring integrity and accuracy. JDL: Contributed to conception or design, drafted the manuscript, critically revised the manuscript, gave final approval, agrees to be accountable for all aspects of work ensuring integrity and accuracy. DJB: Contributed to conception or design, drafted the manuscript, critically revised the manuscript, gave final approval, agrees to be accountable for all aspects of work ensuring integrity and accuracy. DJ: Contributed to conception or design, drafted the manuscript, critically revised the manuscript, gave final approval, agrees to be accountable for all aspects of work ensuring integrity and accuracy. PA: Contributed to conception or design, drafted the manuscript, critically revised the manuscript, gave final approval, agrees to be accountable for all aspects of work ensuring integrity and accuracy. RJH: Contributed to conception or design, drafted the manuscript, critically revised the manuscript, gave final approval, agrees to be accountable for all aspects of work ensuring integrity and accuracy.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to disclose.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent was waived by our IRB due to the fact that the data collected for this study was retrospective and de-identified.
Rights and permissions
About this article
Cite this article
Sujka, J.A., Weaver, K.L., Lim, J.D. et al. A safe and efficacious preventive strategy in the high-risk surgical neonate: cycled total parenteral nutrition. Pediatr Surg Int 34, 1177–1181 (2018). https://doi.org/10.1007/s00383-018-4351-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-018-4351-0