Abstract
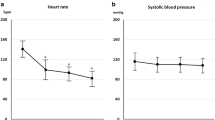
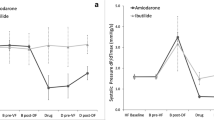
Ivabradine, a bradycardic agent, has been shown to stably reduce patient’s heart rate (HR) in the setting of acute cardiac care. However, an association between atrial fibrillation (AF) risk and acute ivabradine treatment remains a controversial clinical issue, and has not been thoroughly investigated. Bradycardia and abnormal atrial refractoriness induced by ivabradine treatment may enhance vulnerability to AF induction, especially when vagal nerve is concurrently activated. We aimed to experimentally investigate the effects of acute ivabradine treatment with/without concurrent vagal activation on AF inducibility. In 16 anesthetized dogs, cervical vagal nerves were prepared for electrical stimulation (VS). AF induction rate (AFIR) was determined by atrial burst pacing. HR, atrial action potential duration (APD), atrial effective refractory period (ERP), and AFIR were obtained consecutively at baseline, during delivery of VS (VS alone), after intravenous injection of ivabradine 0.5 mg/kg (n = 8, ivabradine group) or saline (n = 8, saline group), and again during VS delivery (drug+VS). In the ivabradine group, ivabradine alone significantly lowered HR compared to baseline, while ivabradine+VS significantly lowered HR compared to VS alone. Contrary to expectations, there were no significant differences in trends of APD, temporal dispersion of APD, ERP, and AFIR between ivabradine and saline groups. Irrespective of whether ivabradine or saline was injected, VS significantly shortened APD and ERP, and increased AFIR. Interestingly, although bradycardia in response to ivabradine injection was more intense than that to VS alone, AFIR was significantly lower after ivabradine injection than during VS alone. We conclude that, despite its intense bradycardic effect, acute ivabradine treatment does not increase AF inducibility irrespective of underlying vagal activity. This study may constitute support for the safety of using ivabradine in the setting of acute cardiac care.





Similar content being viewed by others
References
DiFrancesco D (2010) The role of the funny current in pacemaker activity. Circ Res 106:434–446
Swedberg K, Komajda M, Böhm M, Borer JS, Ford I, Dubost-Brama A, Lerebours G, Tavazzi L, Investigators SHIFT (2010) Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet 376:875–885
Fox K, Ford I, Steg PG, Tendera M, Ferrari R, Investigators BEAUTIFUL (2008) Ivabradine for patients with stable coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): a randomised, double-blind, placebo-controlled trial. Lancet 372:807–816
Fasullo S, Cannizzaro S, Maringhini G, Ganci F, Giambanco F, Vitale G, Pinto V, Migliore G, Torres D, Sarullo FM, Paterna S, Di Pasquale P (2009) Comparison of ivabradine versus metoprolol in early phases of reperfused anterior myocardial infarction with impaired left ventricular function: preliminary findings. J Card Fail 15:856–863
Hidalgo FJ, Anguita M, Castillo JC, Rodríguez S, Pardo L, Durán E, Sánchez JJ, Ferreiro C, Pan M, Mesa D, Delgado M, Ruiz M (2016) Effect of early treatment with ivabradine combined with beta-blockers versus beta-blockers alone in patients hospitalised with heart failure and reduced left ventricular ejection fraction (ETHIC-AHF): a randomised study. Int J Cardiol 217:7–11
Fox K, Ford I, Steg PG, Tardif JC, Tendera M, Ferrari R, SIGNIFY investigators (2015) Bradycardia and atrial fibrillation in patients with stable coronary artery disease treated with ivabradine: an analysis from the SIGNIFY study. Eur Heart J 36:3291–3296
Martin RI, Pogoryelova O, Koref MS, Bourke JP, Teare MD, Keavney BD (2014) Atrial fibrillation associated with ivabradine treatment: meta-analysis of randomised controlled trials. Heart 100:1506–1510
Cammarano C, Silva M, Comee M, Donovan JL, Malloy MJ (2016) Meta-analysis of ivabradine in patients with stable coronary artery disease with and without left ventricular dysfunction. Clin Ther 38:387–395
Abdel-Salam Z, Nammas W (2016) Atrial fibrillation after coronary artery bypass surgery: can ivabradine reduce its occurrence? J Cardiovasc Electrophysiol 27:670–676
Steg P, Lopez-de-Sà E, Schiele F, Hamon M, Meinertz T, Goicolea J, Werdan K, Lopez-Sendon JL, VIVIFY (eValuation of the IntraVenous If inhibitor ivabradine after STsegment elevation mYocardial infarction) investigators (2013) Safety of intravenous ivabradine in acute ST-segment elevation myocardial infarction patients treated with primary percutaneous coronary intervention: a randomized, placebo-controlled, double-blind, pilot study. Eur Heart J Acute Cardiovasc Care 2:270–279
Stieber J, Wieland K, Stöckl G, Ludwig A, Hofmann F (2006) Bradycardic and proarrhythmic properties of sinus node inhibitors. Mol Pharmacol 69:1328–1337
John RM, Kumar S (2016) Sinus node and atrial arrhythmias. Circulation 133:1892–1900
Fedorov VV, Chang R, Glukhov AV, Kostecki G, Janks D, Schuessler RB, Efimov IR (2010) Complex interactions between the sinoatrial node and atrium during reentrant arrhythmias in the canine heart. Circulation 122:782–789
Cacciani F, Zaniboni M (2015) Chronotropic modulation of the source-sink relationship of sinoatrial-atrial impulse conduction and its significance to initiation of AF: a one-dimensional model study. Biomed Res Int 2015:496418
Han J, Millet D, Chizzonitti B, Moe GK (1966) Temporal dispersion of recovery of excitability in atrium and ventricle as a function of heart rate. Am Heart J 71:481–487
Elvan A (2001) Sinoatrial remodeling caused by persistent atrial fibrillation: what is the relationship between postcardioversion sinus node dysfunction and increased atrial vulnerability? J Cardiovasc Electrophysiol 12:807–808
Spach MS, Dolber PC, Heidlage JF (1989) Interactions of inhomogeneities of repolarization with anisotropic propagation in dog atria: a mechanism for both preventing and initiating re-entry. Circ Res 65:1612–1631
Li YD, Ji YT, Zhou XH, Jiang T, Hong YF, Li JX, Xing Q, Xiong J, Yusufuaji Y, Tang BP (2015) Effects of ivabradine on cardiac electrophysiology in dogs with age-related atrial fibrillation. Med Sci Monit 21:1414–1420
Chen PS, Chen LS, Fishbein MC, Lin SF, Nattel S (2014) Role of the autonomic nervous system in atrial fibrillation: pathophysiology and therapy. Circ Res 114:1500–1515
Liu L, Nattel S (1997) Differing sympathetic and vagal effects on atrial fibrillation in dogs: role of refractoriness heterogeneity. Am J Physiol 273:H805–H816
Bonnemeier H, Hartmann F, Wiegand UK, Irmer C, Kurz T, Tölg R, Katus HA, Richardt G (2000) Heart rate variability in patients with acute myocardial infarction undergoing primary coronary angioplasty. Am J Cardiol 85:815–820
Chiladakis JA, Patsouras N, Manolis AS (2003) The Bezold-Jarisch reflex in acute inferior myocardial infarction: clinical and sympathovagal spectral correlates. Clin Cardiol 26:323–328
Uemura K, Inagaki M, Zheng C, Li M, Kawada T, Sugimachi M (2015) A novel technique to predict pulmonary capillary wedge pressure utilizing central venous pressure and tissue Doppler tricuspid/mitral annular velocities. Heart Vessels 30:516–526
Narayan SM, Franz MR (2007) Quantifying fractionation and rate in human atrial fibrillation using monophasic action potentials: implications for substrate mapping. Europace 9:vi89–vi95
Kanki H, Mitamura H, Takatsuki S, Sueyoshi K, Shinagawa K, Sato T, Ogawa S (1998) Postrepolarization refractoriness as a potential anti-atrial fibrillation mechanism of pilsicainide, a pure sodium channel blocker with slow recovery kinetics. Cardiovasc Drugs Ther 12:475–482
Lu Z, Scherlag BJ, Lin J, Niu G, Fung KM, Zhao L, Ghias M, Jackman WM, Lazzara R, Jiang H, Po SS (2008) Atrial fibrillation begets atrial fibrillation: autonomic mechanism for atrial electrical remodeling induced by short-term rapid atrial pacing. Circ Arrhythm Electrophysiol 1:184–192
Colin P, Ghaleh B, Monnet X, Hittinger L, Berdeaux A (2004) Effect of graded heart rate reduction with ivabradine on myocardial oxygen consumption and diastolic time in exercising dogs. J Pharmacol Exp Ther 308:236–240
Zaniboni M, Cacciani F, Lux RL (2014) Beat-to-beat cycle length variability of spontaneously beating guinea pig sinoatrial cells: relative contributions of the membrane and calcium clocks. PLoS One 9:e100242
Zaniboni M, Pollard AE, Yang L, Spitzer KW (2000) Beat-to-beat repolarization variability in ventricular myocytes and its suppression by electrical coupling. Am J Physiol Heart Circ Physiol 278:H677–H687
Franz MR (1983) Long-term recording of monophasic action potentials from human endocardium. Am J Cardiol 51:1629–1634
Ashikaga K, Kobayashi T, Kimura M, Owada S, Sasaki S, Iwasa A, Furukawa K, Motomura S, Okumura K (2006) Effects of amiodarone on electrical and structural remodeling induced in a canine rapid pacing-induced persistent atrial fibrillation model. Eur J Pharmacol 536:148–153
Kabell G, Buchanan LV, Gibson JK, Belardinelli L (1994) Effects of adenosine on atrial refractoriness and arrhythmias. Cardiovasc Res 28:1385–1389
Bode F, Kilborn M, Karasik P, Franz MR (2001) The repolarization-excitability relationship in the human right atrium is unaffected by cycle length, recording site and prior arrhythmias. J Am Coll Cardiol 37:920–925
Tsuboi M, Furukawa Y, Nakajima K, Kurogouchi F, Chiba S (2000) Inotropic, chronotropic, and dromotropic effects mediated via parasympathetic ganglia in the dog heart. Am J Physiol Heart Circ Physiol 279:H1201–H1207
Franz MR (1999) Current status of monophasic action potential recording: theories, measurements and interpretations. Cardiovasc Res 41:25–40
Goldberg JM, Lynn-Johnson MH, Neely B (1981) Use of P wave morphology for inferring pacemaker localization along the sulcus terminalis in the dog. J Electrocardiol 14:115–124
Camm AJ, Lau CP (2003) Electrophysiological effects of a single intravenous administration of ivabradine (S 16257) in adult patients with normal baseline electrophysiology. Drugs R D 4:83–89
Roithinger FX, Karch MR, Steiner PR, SippensGroenewegen A, Lesh MD (1999) The spatial dispersion of atrial refractoriness and atrial fibrillation vulnerability. J Interv Card Electrophysiol 3:311–319
Sugimura S, Kurita T, Kaitani K, Yasuoka R, Miyazaki S (2016) Ectopies from the superior vena cava after pulmonary vein isolation in patients with atrial fibrillation. Heart Vessels 31:1562–1569
Jiang Z, Yin H, He Y, Ma N, Tang M, Liu H, Ding F, Mei J (2015) Efficacy and safety of novel epicardial circumferential left atrial ablation with pulmonary vein isolation in sustained atrial fibrillation. Heart Vessels 30:675–681
Suenari K, Cheng CC, Chen YC, Lin YK, Nakano Y, Kihara Y, Chen SA, Chen YJ (2012) Effects of ivabradine on the pulmonary vein electrical activity and modulation of pacemaker currents and calcium homeostasis. J Cardiovasc Electrophysiol 23:200–206
Zhang Y, Ilsar I, Sabbah HN, Ben David T, Mazgalev TN (2009) Relationship between right cervical vagus nerve stimulation and atrial fibrillation inducibility: therapeutic intensities do not increase arrhythmogenesis. Heart Rhythm 6:244–250
De Ferrari GM, Mazzuero A, Agnesina L, Bertoletti A, Lettino M, Campana C, Schwartz PJ, Tavazzi L (2008) Favourable effects of heart rate reduction with intravenous administration of ivabradine in patients with advanced heart failure. Eur J Heart Fail 10:550–555
Colin P, Ghaleh B, Monnet X, Su J, Hittinger L, Giudicelli JF, Berdeaux A (2003) Contributions of heart rate and contractility to myocardial oxygen balance during exercise. Am J Physiol Heart Circ Physiol 284:H676–H682
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
This work was supported by JSPS KAKENHI Grant No. 15K01307, and also by Practical Research Project for Life-Style related Diseases including Cardiovascular Diseases and Diabetes Mellitus from Japan Agency for Medical Research and Development.
Rights and permissions
About this article
Cite this article
Uemura, K., Inagaki, M., Zheng, C. et al. Acute ivabradine treatment reduces heart rate without increasing atrial fibrillation inducibility irrespective of underlying vagal activity in dogs. Heart Vessels 32, 484–494 (2017). https://doi.org/10.1007/s00380-016-0922-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-016-0922-y