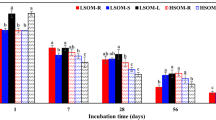
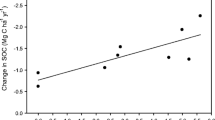
Abstract
Agricultural soils receive large amounts of anthropogenic nitrogen (N), which directly and indirectly affect soil organic matter (SOM) stocks and CO2 fluxes. However, our current understanding of mechanisms on how N fertilization affects SOM pools of various ages and turnover remains poor. The δ13C values of SOM after wheat (C3)-maize (C4) vegetation change were used to calculate the contribution of C4-derived rhizodeposited C (rhizo-C) and C3-derived SOM pools, i.e., rhizo-C and SOM. Soil (Ap from Haplic Luvisol) sampled from maize rhizosphere was incubated over 56 days with increasing N fertilization (four levels up to 300 kg N ha−1), and CO2 efflux and its δ13C were measured. Nitrogen fertilization decreased CO2 efflux by 27–42% as compared to unfertilized soil. This CO2 decrease was mainly caused by the retardation of SOM (C3) mineralization. Microbial availability of rhizo-C (released by maize roots within 4 weeks) was about 10 times higher than that of SOM (older than 4 weeks). Microbial biomass and dissolved organic C remained at the same level with increasing N. However, N fertilization increased the relative contribution of rhizo-C to microbial biomass by two to five times and to CO2 for about two times. This increased contribution of rhizo-C reflects strongly accelerated microbial biomass turnover by N addition. The decomposition rate of rhizo-C was 3.7 times faster than that of SOM, and it increased additionally by 6.5 times under 300 kg N ha−1 N fertilization. This is the first report estimating the turnover and incorporation of very recent rhizo-C (4 weeks old) into soil C pools and shows that the turnover of rhizo-C was much faster than that of SOM. We conclude that the contribution of rhizo-C to CO2 and to microbial biomass is highly dependent on N fertilization. Despite acceleration of rhizo-C turnover, the increased N fertilization facilitates C sequestration by decreasing SOM decomposition.





Similar content being viewed by others
References
Amelung W, Brodowski S, Sandhage-Hofmann A, Bol R (2008) Combining biomarker with stable isotope analyses for assessing the transformation and turnover of soil organic matter. Adv Agron 100:155–250
Balesdent J, Mariotti A (1996) Measurement of soil organic matter turnover using 13C natural abundance. In: Boutton TW, Yamasaki S (eds) Mass Spectrometry of Soils; Boutton, T.W., Yamasaki, S., Eds.; New York,. Marcel Dekker, Inc., New York, pp 47–82
Blagodatskaya E, Yuyukina T, Blagodatsky S, Kuzyakov Y (2011) Turnover of soil organic matter and of microbial biomass under C3-C4 vegetation change: consideration of 13C fractionation and preferential substrate utilization. Soil Biol Biochem 43:159–166. doi:10.1016/j.soilbio.2010.09.028
Blagodatskaya E, Blagodatsky S, Anderson TH, Kuzyakov Y (2014) Microbial growth and carbon use efficiency in the rhizosphere and root-free soil. PLoS One 9:e93282. doi:10.1371/journal.pone.0093282
Blagodatsky SA, Yevdokimov IV, Larionova AA, Richter J (1998) Microbial growth in soil and nitrogen turnover: model calibration with laboratory data. Soil Biol Biochem 30:1757–1764. doi:10.1016/S0038-0717(98)00029-7
Bol R, Poirier N, Balesdent J, Gleixner G (2009) Molecular turnover time of soil organic matter in particle-size fractions of an arable soil. Rapid Commun Mass Spectrom 23:2551–2558. doi:10.1002/rcm.4124
Burton AJ, Pregitzer KS, Crawford JN, Zogg GP, Zak DR (2004) Simulated chronic NO3 − deposition reduces soil respiration in northern hardwood forests. Glob Chang Biol 10:1080–1091. doi:10.1111/j.1365-2486.2004.00737.x
Cardon ZG, Hungate BA, Cambardella CA, Chapin FS, Field CB, Holland EA, Mooney HA (2001) Contrasting effects of elevated CO2 on old and new soil carbon pools. Soil Biol Biochem 33:365–373. doi:10.1016/S0038-0717(00)00151-6
Chen R, Senbayram M, Blagodatsky S, Myachina O, Dittert K, Lin X, Blagodatskaya E, Kuzyakov Y (2014) Soil C and N availability determine the priming effect: microbial N mining and stoichiometric decomposition theories. Glob Chang Biol 20:2356–2367. doi:10.1111/gcb.12475
Cheng W (1999) Rhizosphere feedbacks in elevated CO2. Tree Physiol 19:313–320. doi:10.1093/treephys/19.4-5.313
Cleveland CC, Townsend AR (2006) Nutrient additions to a tropical rain forest drive substantial soil carbon dioxide losses to the atmosphere. Proc Natl Acad Sci U S A 103:10316–10321. doi:10.1073/pnas.0600989103
Dijkstra FA, Carrillo Y, Pendall E, Morgan JA (2013) Rhizosphere priming: a nutrient perspective. Front Microbiol 4:1–8. doi:10.3389/fmicb.2013.00216
Dou X, He P, Cheng X, Zhou W (2016) Long-term fertilization alters chemically-separated soil organic carbon pools: based on stable C isotope analyses. Sci Rep 6:19061. doi:10.1038/srep19061
Esperschütz J, Buegger F, Winkler JB, Munch JC, Schloter M, Gattinger A (2009) Microbial response to exudates in the rhizosphere of young beech trees (Fagus sylvatica L.) after dormancy. Soil Biol Biochem 41:1976–1985. doi:10.1016/j.soilbio.2009.07.002
Fischer H, Kuzyakov Y (2010) Sorption, microbial uptake and decomposition of acetate in soil: transformations revealed by position-specific 14C labeling. Soil Biol Biochem 42:186–192. doi:10.1016/j.soilbio.2009.10.015
Fischer H, Ingwersen J, Kuzyakov Y (2010) Microbial uptake of low-molecular-weight organic substances out-competes sorption in soil. Eur J Soil Sci 61:504–513. doi:10.1111/j.1365-2389.2010.01244.x
Fischlin A, Midgley GF, Price JT (2007) Ecosystems, their properties, goods and services. In: Parry ML, Canziani OF, Palutikof JP, van der Linden PJ, Hanson CE (eds) Climate Change 2007: Impacts, adaptation and vulnerability. Cambridge University Press, Cambridge, pp 211–272
Flessa H, Ludwig B, Heil B, Merbach W (2000) The origin of soil organic C, dissolved organic C and respiration in a long-term maize experiment in Halle, Germany, determined by 13C natural abundance. J Plant Nutr Soil Sci 163:157–163. doi:10.1002/(SICI)1522-2624(200004)163:2<157::AID-JPLN157>3.0.CO;2-9
Fog K (1988) The effect of added nitrogen on the rate of decomposition of organic matter. Biol Rev 63:433–462. doi:10.1111/j.1469-185X.1988.tb00725.x
Fontaine S, Mariotti A, Abbadie L (2003) The priming effect of organic matter: a question of microbial competition? Soil Biol Biochem 35:837–843. doi:10.1016/S0038-0717(03)00123-8
Formánek P, Ambus P (2004) Assessing the use of δ13C natural abundance in separation of root and microbial respiration in a Danish beech (Fagus sylvatica L.) forest. Rapid Commun Mass Spectrom 18:897–902. doi:10.1002/rcm.1424
Galloway JN, Townsend AR, Erisman JW, Bekunda M, Cai Z, Freney JR, Martinelli LA, Seitzinger SP, Sutton MA (2008) Transformation of the nitrogen cycle: recent trends, questions, and potential solutions. Science 320:889–892. doi:10.1126/science.1136674
González Polo M, Kowaljow E, Castán E, Sauzet O, Mazzarino MJ (2015) Persistent effect of organic matter pulse on a sandy soil of semiarid Patagonia. Biol Fertil Soils 51:241–249. doi:10.1007/s00374-014-0961-4
Grulke NE, Andersen CP, Fenn ME, Miller PR (1998) Ozone exposure and nitrogen deposition lowers root biomass of ponderosa pine in the San Bernardino Mountains, California. Environ Pollut 103:63–73. doi:10.1016/S0269-7491(98)00130-4
Hobbie SE, Eddy WC, Buyarski CR, Adair EC, Ogdahl ML, Weisenhorn P (2012) Response of decomposing litter and its microbial community to multiple forms of nitrogen enrichment. Ecol Monogr 82:389–405. doi:10.1890/11-1600.1
Insam H, Haselwandter K (1989) Metabolic quotient of the soil microflora in relation to plant succession. Oecologia 79:174–178. doi:10.1007/BF00388474
Janssens IA, Dieleman W, Luyssaert S, Subke JA, Reichstein M, Ceulemans R, Ciais P, Dolman AJ, Grace J, Matteucci G, Papale D (2010) Reduction of forest soil respiration in response to nitrogen deposition. Nat Geosci 3:315–322. doi:10.1038/ngeo844
Joergensen RG (1996) The fumigation-extraction method to estimate soil microbial biomass: calibration of the k EC value. Soil Biol Biochem 28:25–31. doi:10.1016/0038-0717(95)00102-6
Kuzyakov Y (2011) How to link soil C pools with CO2 fluxes? Biogeosciences 8:1523–1537. doi:10.5194/bg-8-1523-2011
Kuzyakov Y, Hill PW, Jones DL (2007) Root exudate components change litter decomposition in a simulated rhizosphere depending on temperature. Plant Soil 290:293–305. doi:10.1007/s11104-006-9162-8
Ladha JK, Reddy CK, Padre AT, van Kessel C (2011) Role of nitrogen fertilization in sustaining organic matter in cultivated soils. J Environ Qual 40:1756–1766. doi:10.2134/jeq2011.0064
Lal R (2006) Enhancing crop yields in the developing countries through restoration of the soil organic carbon pool in agricultural lands. L Degrad Dev 17:197–209. doi:10.1002/ldr.696
Liang Q, Chen H, Gong Y, Fan M, Yang H, Lal R, Kuzyakov Y (2012) Effects of 15 years of manure and inorganic fertilizers on soil organic carbon fractions in a wheat-maize system in the North China Plain. Nutr Cycl Agroecosystems 92:21–33. doi:10.1007/s10705-011-9469-6
Lin J, Zhu B, Cheng W (2015) Decadally cycling soil carbon is more sensitive to warming than faster-cycling soil carbon. Glob Chang Biol 21:4602–4612. doi:10.1111/gcb.13071
Liu L, Greaver TL (2010) A global perspective on belowground carbon dynamics under nitrogen enrichment. Ecol Lett 13:819–828. doi:10.1111/j.1461-0248.2010.01482.x
Luo Y, Zang H, Yu Z, Chen Z, Gunina A, Kuzyakov Y, Xu J, Zhang K, Brookes PC (2017) Priming effects in biochar enriched soils using a three-source-partitioning approach: 14C labelling and 13C natural abundance. Soil Biol Biochem 106:28–35. doi:10.1016/j.soilbio.2016.12.006
von Lützow M, Kögel-Knabner I, Ekschmitt K, Flessa H, Guggenberger G, Matzner E, Marschner B (2007) SOM fractionation methods: relevance to functional pools and to stabilization mechanisms. Soil Biol Biochem 39:2183–2207. doi:10.1016/j.soilbio.2007.03.007
Mulvaney RL, Khan SA, Ellsworth TR (2009) Synthetic nitrogen fertilizers deplete soil nitrogen: a global dilemma for sustainable cereal production. J Environ Qual 38:2295. doi:10.2134/jeq2008.0527
Pausch J, Kuzyakov Y (2012) Soil organic carbon decomposition from recently added and older sources estimated by δ13C values of CO2 and organic matter. Soil Biol Biochem 55:40–47. doi:10.1016/j.soilbio.2012.06.007
Pelz O, Abraham WR, Saurer M, Siegwolf R, Zeyer J (2005) Microbial assimilation of plant-derived carbon in soil traced by isotope analysis. Biol Fertil Soils 41:153–162. doi:10.1007/s00374-004-0826-3
Phillips DL, Gregg JW (2001) Erratum: uncertainty in source partitioning using stable isotopes (Oecologia (2001) 127:2 (171–179)). Oecologia 128:304. doi: 10.1007/s004420100723
Ramirez KS, Craine JM, Fierer N (2012) Consistent effects of nitrogen amendments on soil microbial communities and processes across biomes. Glob Chang Biol 18:1918–1927. doi:10.1111/j.1365-2486.2012.02639.x
Riggs CE, Hobbie SE, Bach EM, Hofmockel KS, Kazanski CE (2015) Nitrogen addition changes grassland soil organic matter decomposition. Biogeochemistry 125:203–219. doi:10.1007/s10533-015-0123-2
Schlesinger WH (2009) On the fate of anthropogenic nitrogen. Proc Natl Acad Sci 106:203–208. doi:10.1073/pnas.0810193105
Schneckenberger K, Kuzyakov Y (2007) Carbon sequestration under Miscanthus in sandy and loamy soils estimated by natural 13C abundance. J Plant Nutr Soil Sci 170:538–542. doi:10.1002/jpln.200625111
Treseder KK (2008) Nitrogen additions and microbial biomass: a meta-analysis of ecosystem studies. Ecol Lett 11:1111–1120. doi:10.1111/j.1461-0248.2008.01230.x
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19:703–707. doi:10.1016/0038-0717(87)90052-6
Vogel C, Heister K, Buegger F, Tanuwidjaja I, Haug S, Schloter M, Kögel-Knabner I (2015) Clay mineral composition modifies decomposition and sequestration of organic carbon and nitrogen in fine soil fractions. Biol Fertil Soils 51:427–442. doi:10.1007/s00374-014-0987-7
Wardle DA, Ghani A (1995) A critique of the microbial metabolic quotient (qCO2) as a bioindicator of disturbance and ecosystem development. Soil Biol Biochem 27:1601–1610. doi:10.1016/0038-0717(95)00093-T
Werth M, Kuzyakov Y (2008) Root-derived carbon in soil respiration and microbial biomass determined by 14C and 13C. Soil Biol Biochem 40:625–637. doi:10.1016/j.soilbio.2007.09.022
Werth M, Kuzyakov Y (2010) 13C fractionation at the root-microorganisms-soil interface: a review and outlook for partitioning studies. Soil Biol Biochem 42:1372–1384. doi:10.1016/j.soilbio.2010.04.009
Wu J, Joergensen RG, Pommerening B, Chaussod R, Brookes PC (1990) Measurement of soil microbial biomass C by fumigation-extraction—an automated procedure. Soil Biol Biochem 22:1167–1169. doi:10.1016/0038-0717(90)90046-3
Yuan H, Zhu Z, Liu S, Ge T, Jing H, Li B, Liu Q, Lynn TM, Wu J, Kuzyakov Y (2016) Microbial utilization of rice root exudates: 13C labeling and PLFA composition. Biol Fertil Soils 52:615–627. doi:10.1007/s00374-016-1101-0
Zang H, Yang X, Feng X, Qian X, Hu Y, Ren C, Zeng Z (2015) Rhizodeposition of nitrogen and carbon by mungbean (Vigna radiata L.) and its contribution to intercropped oats (Avena nuda L.) PLoS One. doi:10.1371/journal.pone.0121132
Zang H, Wang J, Kuzyakov Y (2016) N fertilization decreases soil organic matter decomposition in the rhizosphere. Appl Soil Ecol 108:47–53. doi:10.1016/j.apsoil.2016.07.021
Acknowledgements
We thank the China Scholarship Council for funding to Huadong Zang in Germany. This study was supported by Deutsche Forschungsgemeinschaft (DFG; KU-1184/13-2) within the Research Unit: Soil Food Webs. EB’s participation was supported by the Russian Science Foundation (project no. 14-14-00625). The isotopic analyses were performed at the Kompetenzzentrum Stabile Isotope (KOSI), Goettingen. The authors also would like to thank Karin Schmidt and Anita Kriegel for their laboratory assistance.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Fig. S1
(DOCX 479 kb)
Rights and permissions
About this article
Cite this article
Zang, H., Blagodatskaya, E., Wang, J. et al. Nitrogen fertilization increases rhizodeposit incorporation into microbial biomass and reduces soil organic matter losses. Biol Fertil Soils 53, 419–429 (2017). https://doi.org/10.1007/s00374-017-1194-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-017-1194-0