Abstract
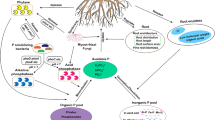
Alkaline phosphomonoesterase (ALP) mainly originates from soil microbial secretion and plays a crucial role in the turnover of soil phosphorus (P). To examine the response of ALP-encoding microbial communities (analysed for the biomarker of the ALP gene, phoD) of soils and derivative soil fractions to different fertilisation regimes, soil samples were collected from a long-term experimental field (over 35 years). The different organic P (Po) pools of soil fractions and the ALP activity of soil were also determined. Compared with chemical-only fertilised soils, the ALP activity was 232–815% higher in organic-amended soils, and the highest enzyme activity was observed in the organic-only fertilised treatment. The abundance of the phoD gene harbouring in soil fractions, determined by quantitative PCR (qPCR), was affected by different fertilisations. The highest abundance of the phoD gene was generally detected in the 2–63-μm-sized fraction (silt), but most phoD-encoding microbial species were associated to the 0.1–2-μm-sized fraction (clay) in the chemical-only fertilised soil. The contents of labile Po (LPo), moderately labile Po (MLPo) and fulvic acid-associated Po (FAPo) were significantly correlated with the phoD gene abundance, whereas only LPo content was significantly correlated with the ALP activity. The dominant phoD-encoding phylas were Actinobacteria and Proteobacteria, according to a high-throughput sequencing. Bradyrhizobium, a N2-fixer identified as a phoD-encoding genus, showed the highest abundance in fertilised soils. The abundance of Bradyrhizobium, Streptomyces, Modestobacter, Lysobacter, Frankia and Burkholderia increased with the organic-only amendment and was significantly correlated with the ALP activity. According to structure equation models (SEM), pH and LPo content significantly and directly affected the ALP activity; the soil organic C (Corg) content was related to composition and abundances of phoD-harbouring microbial communities; since both microbial properties were correlated to the ALP activity, the Corg content was indirectly related to the ALP activity. In conclusion, soil management practices can be used to optimise the contents of soil available P and the organic P with regulation of soil ALP activity and the community composition of corresponding microbes.






Similar content being viewed by others
References
Acuña JJ, Durán P, Lagos LM (2016) Bacterial alkaline phosphomonoesterase in the rhizospheres of plants grown in chilean extreme environments. Biol Fert Soils 52:1–11
Alniemi TS, Summers ML, Elkins JG, Kahn ML, Mcdermott TR (1997) Regulation of the phosphate stress response in rhizobium meliloti by phoB. Appl Environ Microb 63:4978–4981
Bakker MG, Otto-Hanson L, Lange AJ, Bradeen JM, Kinkel LL (2013) Plant monocultures produce more antagonistic soil streptomyces, communities than high-diversity plant communities. Soil Biol Biochem 65:304–312
Bowman RA, Cole CV (1978) An exploratory method for fractionation of organic phosphorus from grassland soils. Soil Sci 125:95–101
Brockett BFT, Prescott CE, Grayston SJ (2012) Soil moisture is the major factor influencing microbial community structure and enzyme activities across seven biogeoclimatic zones in western Canada. Soil Biol Biochem 4:9–20
Chaudhry V, Rehman A, Mishra A, Chauhan PS, Nautiyal CS (2012) Changes in bacterial community structure of agricultural land due to long-term organic and chemical amendments. Microb Ecol 64:450–460
Chen H (2003) Phosphatase activity and P fractions in soils of an 18-year-old Chinese fir (Cunninghamia lanceolata) plantation. Forest Ecol Manag 178:301–310
Chhabra S, Brazil D, Morrissey J, Burke J, O’Gara F, Dowling DN (2012) Fertilization management affects the alkaline phosphatase bacterial community in barley rhizosphere soil. Biol Fert Soils 49:31–39
Chu H, Grogan P (2010) Soil microbial biomass, nutrient availability and nitrogen mineralization potential among vegetation-types in a low arctic tundra landscape. Plant Soil 329:411–420
Chung H, Grove JH, Six J (2008) Indications for soil carbon saturation in a temperate agroecosystem. Soil Sci Soc Am J 72:1132–1139
Cordell D, Drangert JO, White S (2009) The story of phosphorus: global food security and food for thought. Global Environ Chang 19:292–305
Cui H, Zhou Y, Gu Z, Zhu H, Fu S, Yao Q (2015) The combined effects of cover crops and symbiotic microbes on phosphatase gene and organic phosphorus hydrolysis in subtropical orchard soils. Soil Biol Biochem 82:119–126
Dalal RC (1978) Organic phosphorus. Adv Agron 29:83–117
Dick RP, Rasmussen PE, Kerle EA (1988) Influence of long-term residue management on soil enzyme activities in relation to soil chemical properties of a wheat-fallow system. Biol Fert Soils 6:159–164
Dick WA, Cheng L, Wang P (2000) Soil acid and alkaline phosphatase activity as pH adjustment indicators. Soil Biol Biochem 32:1915–1919
Edgar RC (2013) UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods 10:996–998
Fraser T, Lynch DH, Entz MH, Dunfield KE (2015a) Linking alkaline phosphatase activity with bacterial phoD gene abundance in soil from a long-term management trial. Geoderma 257:115–122
Fraser TD, Lynch DH, Bent E, Entz MH, Dunfield KE (2015b) Soil bacterial phoD, gene abundance and expression in response toapplied phosphorus and long-term management. Soil Biol Biochem 88:137–147
Grace JB (2006) Structural equation modeling and natural systems. Cambridge University Press, Cambridge
Hemkemeyer M, Christensen BT, Martens R, Tebbe CC (2015) Soil particle size fractions harbour distinct microbial communities and differ in potential for microbial mineralisation of organic pollutants. Soil Biol Biochem 90:255–265
Heuck C, Weig A, Spohn M (2015) Soil microbial biomass C:N:P stoichiometry and microbial use of organic phosphorus. Soil Biol Biochem 85:119–129
Huang S, Peng X, Huang Q (2010) Soil aggregation and organic carbon fractions affected by long-term fertilization in a red soil of subtropical China. Geoderma 154:364–369
Kageyama H, Tripathi K, Rai AK, Chaum S, Waditee-Sirisattha R, Takabe T (2011) An alkaline phosphatase/phosphodiesterase, PhoD, induced by salt stress and secreted out of the cells of Aphanothece halophytica, a halotolerant cyanobacterium. Appl Environ Microb 77:5178–5183
Kathuria S, Martiny AC (2011) Prevalence of a calcium-based alkaline phosphatase associated with the marine cyanobacterium prochlorococcus and other ocean bacteria. Environ Microb 13:74–83
Lagos LM, Acuna JJ, Maruyama F, Ogram A, Mora MDLL, Jorquera MA (2016) Effect of phosphorus addition on total and alkaline phosphomonoesterase-harboring bacterial populations in ryegrass rhizosphere microsites. Biol Fert Soils 52:1–13
Letunic I, Bork P (2006) Interactive tree of life (iTOL): an online tool for phylogenetic tree display and annotation. Bioinformatics 23:127–128
Ling N, Sun YM, Guo JJ, Zhu P, Peng C, Yu GH, Ran W, Guo SW, Shen QR (2014) Response of the bacterialdiversity and soil enzyme activity in particle-size fractions of Mollisol after different fertilization in a long-term experiment. Biol Fert Soils 50:901–911
Marklein AR, Houlton BZ (2012) Nitrogen inputs accelerate phosphorus cycling rates across a wide variety of terrestrial ecosystems. New Phytol 193:696–704
Martin M (2011) Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet Journal 17(1). doi:10.14806/ej.17.1.200
Nannipieri P, Giagnoni L, Landi L, Renella G (2011) Role of phosphatase enzymes in soil. In: Bünemann EK et al (eds) Phosphorus in action, soil biology, vol 26, pp 215–243
Nannipieri P, Giagnoni L, Renella G, Puglisi E, Ceccanti B, Masciandaro G, Fornasier F, Moscatelli MC, Marinari S (2012) Soil enzymology: classical and molecular approaches. Biol Fertil Soils 48:743–762
Nicolás C, Hernández T, García C (2012) Organic amendments as strategy to increase organic matter in particle-size fractions of a semi-arid soil. Appl Soil Ecol 57:50–58
Ragot SA, Huguenin-Elie O, Kertesz MA, Frossard E, Bünemann EK (2015) Total and active microbial communities and phoD as affected by phosphate depletion and pH in soil. Plant Soil 408:15–30
Richardson AE (2001) Prospects for using soil microorganisms to improve the acquisition of phosphorus by plants. Aust J Plant Physiol 28:897–906
Saha S, Prakash V, Kundu S, Kumar N, Lal Mina B (2008) Soil enzymatic activity as affected by long term application of farm yard manure and mineral fertilizer under rainfed soybean-wheat system in N-W Himalaya. Eur J Soil Biol 44:309–315
Sakurai M, Wasaki J, Tomizawa Y, Shinano T, Osaki M (2008) Analysis of bacterial communities on alkaline phosphatase genes in soil supplied with organic matter. Soil Sci Plant Nutr 54:62–71
Schlatter DC, Bakker MG, Bradeen JM, Kinkel LL (2015) Plant community richness and microbial interactions structure bacterial communities in soil. Ecology 96:134–142
Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, Sahl JW, Stres B, Thallinger GG, Van Horn DJ, Weber CF (2009) Introducing mothur: opensource, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microb 75:7538–7541
Senwo ZN, Ranatunga TD, Tazisong IA, Taylor RW, He Z (2007) Phosphatase activity of ultisols and relationship to soil fertility indices. J Food Agric Environ 5(1):262–266
Slazak A, Freese D, Matos EDS, Hüttl RF (2010) Soil organic phosphorus fraction in pine–oak forest stands in northeastern Germany. Geoderma 158:156–162
Smart JB, Dilworth MJ, Robson AD (1984) Effect of phosphorus supply on phosphate uptake and alkaline phosphatase activity in rhizobia. Arch Microbiol 140:281–286
Spohn M, Kuzyakov Y (2013a) Distribution of microbial- and root-derived phosphatase activities in the rhizosphere depending on P availability and C allocatione coupling soil zymography with 14C imaging. Soil Biol Biochem 67:106–113
Spohn M, Kuzyakov Y (2013b) Phosphorus mineralization can be driven by microbial need for carbon. Soil Biol Biochem 61:69–75
Spohn M, Treichel NS, Cormann M, Schloter M, Fischer D (2015) Distribution of phosphatase activity and various bacterial phyla in the rhizosphere of Hordeum vulgare L. depending on P availability. Soil Biol Biochem 89:44–51
Steenbergh AK, Bodelier PLE, Hoogveld HL, Slomp CP, Laanbroek HJ (2011) Phosphatases relieve carbon limitation of microbial activity in Baltic Sea sediments along a redox-gradient. Limnol Oceanogr 56:2018–2026
Stemmer M, Gerzabek MH, Kandeler E (1998) Organic matter and enzyme activity in particle-size fractions of soils obtained after low-energy sonication. Soil Biol Biochem 30:9–17
Tabatabai MA, Bremner JM (1969) Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol Biochem 1:301–307
Tan H, Matthieu B, Mooij MJ, Rice O, Morrissey JP, Dobson A, Griffiths B, O'Gara F (2013) Long-term phosphorus fertilisation increased the diversity of the total bacterial community and the phoD phosphorus mineraliser group in pasture soils. Biol Fert Soils 49:661–672
Tarafdar JC, Claassen N (1988) Organic phosphorus compounds as a phosphorus source for higher plants through the activity of phosphatase produced by plant roots and microorganisms. Biol Fert Soils 5:308–312
Tarafdar JC, Jungk A (1987) Phosphatase activity in the rhizosphere and its relation to the depletion of soil organic phosphorus. Biol Fert Soils 3(4):199–204
Tiecher T, Santos DR, Calegari A (2012) Soil organic phosphorus forms under different soil management systems and winter crops, in a long term experiment. Soil Till Res 124:57–67
Waldrip HM, He Z, Erich MS (2011) Effects of poultry manure amendment on phosphorus uptake by ryegrass, soil phosphorus fractions and phosphatase activity. Biol Fert Soils 47(4):407–418
Wang Y, Zhang F, Marschner P (2012) Soil pH is the main factor influencing growth and rhizosphere properties of wheat following different pre-crops. Plant Soil 360:271–286
Wei K, Chen Z, Zhu A, Zhang J, Chen L (2014) Application of 31P NMR spectroscopy in determining phosphatase activities and P composition in soil aggregates influenced by tillage and residue management practices. Soil Till Res 138:35–43
Welsh C, Tenuta M, Flaten DN, Thiessen-Martens JR, Entz MH (2009) High yielding organic crop management decreases plant-available but not recalcitrant soil phosphorus. Agron J 101:1027–1035
Withers PJA, Haygarth PM (2007) Agriculture, phosphorus and eutrophication: a European perspective. Soil Use Manage 23:1–4
Wu JR, Shien JH, Shieh HK, Hu CC, Gong SR, Chen LY, Chang PC (2007) Cloning of the gene and characterization of the enzymatic properties of the monomeric alkaline phosphatase (PhoX) from Pasteurella multocida strain X-73. FEMS Microbiol Lett 267:113–120
Xun W, Zhao J, Xue C, Zhang G, Ran W, Wang B (2016) Significant alteration of soil bacterial communities and organic carbon decomposition by different long-term fertilization management conditions of extremely low-productivity arable soil in South China. Environ Microb 18:1907–1917
Yagil EZRA, Beacham IR (1975) Uptake of adenosine 50-monophosphate by Escherichia coli. J Bacteriol 121:401–405
Yu HY, Ding WX, Luo JF, Donnison A, Zhang JB (2012) Long-term effect of compost and inorganic fertilizer on activities of carbon-cycle enzymes in aggregates of an intensively cultivated sandy loam. Soil Use Manage 28:347–360
Zhang A, Chen Z, Zhang G, Chen L, Wu Z (2012) Soil phosphorus composition determined by 31P NMR spectroscopy and relative phosphatase activities influenced by land use. Eur J Soil Biol 52:73–77
Zhang J, Kobert K, Flouri T, Stamatakis A (2013) PEAR: a fast and accurate Illumina paired-end reAd mergeR. Bioinformatics 30:614–620
Zhang G, Chen Z, Zhang A, Chen L, Wu Z, Ma X (2014a) Phosphorus composition and phosphatase activities in soil affected by long-term application of pig manure and inorganic fertilizers. Commun Soil Sci Plan 45:1866–1876
Zhang WL, Xu AG, Zhang RL, Ji HJ (2014b) Review of soil classification and revision of China soil classification system. Sci Agric Sin 47:3214–3230
Zhong W, Gu T, Wang W, Zhang B, Lin X, Huang Q, Shen W (2010) The effects of mineral fertilizer and organic manure on soil microbial community and diversity. Plant Soil 326:511–522
Zimmerman AE, Martiny A, Allison SD (2013) Microdiversity of extracellular enzyme genes among sequenced prokaryotic genomes. ISME J 7:1187–1199
Acknowledgements
This work was financially supported by the China Science and Technology Ministry (2015CB150500, 2013CB127403), Youth Innovation Fund of Anhui Academy of Agricultural Sciences (15B0204) and Jiangsu Science and Technology Department (BK20150059).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 356 kb)
Rights and permissions
About this article
Cite this article
Luo, G., Ling, N., Nannipieri, P. et al. Long-term fertilisation regimes affect the composition of the alkaline phosphomonoesterase encoding microbial community of a vertisol and its derivative soil fractions. Biol Fertil Soils 53, 375–388 (2017). https://doi.org/10.1007/s00374-017-1183-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-017-1183-3