Abstract
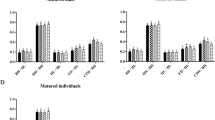
Triplophysa rosa is a typical species of cave-dwelling fish distributed throughout Wulong County, Chongqing, China. This study aimed to test whether T. rosa has a low metabolic level as a cave species and how the metabolic rate of this fish responds to light stimulation. The whole body and organ (including brain, heart, and liver) oxygen consumption rates (\({\dot {m}_{{{\text{O}}_{\text{2}}}}}\)) and several blood parameters related to oxygen carrying were compared between T. rosa acclimated in constant dark and those in regular photoperiod conditions. No significant changes in any variables were observed between the regular photoperiod fish and the dark fish, suggesting that the metabolic consumption of T. rosa is not light sensitive, which may be attributed to the highly degraded eyes of this cave species. The average mass-specific resting \({\dot {m}_{{{\text{O}}_{\text{2}}}}}\) of T. rosa was 38.3 mgO2 kg− 1 h− 1 and was lower than many other fish species. One possible explanation for the low metabolic level of T. rosa can be due to its highly degraded eyes and small brain size. Whole-organ \({\dot {m}_{{{\text{O}}_{\text{2}}}}}\) of the brain, heart, and liver were on average responsible for 8.18%, 3.55%, and 8.61% of the body resting \({\dot {m}_{{{\text{O}}_{\text{2}}}}}\), respectively. Both heart mass and liver mass increased with increasing body mass; however, brain mass did not correlate with body mass. Maintaining a small brain size throughout ontogeny suggests energy-saving advantages for this cave species.







Similar content being viewed by others
Abbreviations
- \({\dot {m}_{{{\text{O}}_{\text{2}}}}}\) :
-
Oxygen consumption rate
- Hb:
-
Hemoglobin concentration
- RBC:
-
Red blood cell
- RBCC:
-
Red blood cell count
- L :
-
Body length
- M :
-
Body mass
References
Aiello LC, Wheeler P (1995) The expensive-tissue hypothesis: the brain and the digestive system in human and primate evolution. Curr Anthropol 36:199–221
Boeuf G, Le Bail PY (1999) Does light have an influence on fish growth? Aquaculture 177:129–152
Brison LL (2001) Experimental analysis of metabolic adaptation of Cottus carolinae in response to photoperiod and food availability. Honors Theses, Southern Illinois University, Carbondale
Chen XY, Yang JX (2005) Triplophysa rosa sp. nov.: a new blind loach from China. J Fish Biol 66:599–608
Childress JJ (1995) Are there physiological and biochemical adaptations of metabolism in deep-sea animals? Trends Ecol Evol 10:30–36
Clarke A, Johnston NM (1999) Scaling of metabolic rate with body mass and temperature in teleost fish. J Anim Ecol 68(5):893–905
Dacie JV, Lewis SN (1984) Practical Haematology, 5th edn. Churchill Livingstone, Edinburgh
Davison J (1955) Body weight, cell surface and metabolic rate in anuran Amphibia. Biol Bull 109:407–419
Duston J, Saunders RL (1990) The entrainment role of photoperiod on hypoosmoregulatory and growth-related aspects of smolting in Atlantic salmon (Salmo salar). Can J Zool 68:707–715
Gillooly JF, Mccoy MW (2014) Brain size varies with temperature in vertebrates. PeerJ 2:e301
Glazier DS (2010) A unifying explanation for diverse metabolic scaling in animals and plants. Biol Rev 85:111–138
Goolish EM, Adelman IR (1987) Tissue-specific cytochrome oxidase activity in largemouth bass: the metabolic costs of feeding and growth. Physiol Zool 60:454–464
Goolish EM, Adelman IR (1988) Tissue-specific allometry of an aerobic respiratory enzyme in a large and a small species of cyprinid (Teleostei). Can J Zool 66:2199–2208
He G, He L, Xu Y, Zhang Z, Li X (2008) Study on germplasm characters of Triplophysa xiangxiensis. Freshw Fish 38:64–67
Hochachka PW (2003) Intracellular convection, homeostasis and metabolic regulation. J Exp Biol 206:2001–2009
Huang J (2012) Comparative studies on the gross anatomy and histological structure of the central nervous system between. In: Triplophysa bleekeri and Triplophysa rosa. Southwest University, Chongqing
Hüppop K (1986) Oxygen consumption of Astyanax fasciatus (Characidae, Pisces): a comparison of epigean and hypogean populations. Environ Biol Fish 17:299–308
Jayasundara N, Kozal JS, Arnold MC, Chan SS, Giulio RT (2015) High-throughput tissue bioenergetics analysis reveals identical metabolic allometric scaling for teleost hearts and whole organisms. PLoS One 10:e0137710
Killen SS, Atkinson D, Glazier DS (2010) The intraspecific scaling of metabolic rate with body mass in fishes depends on lifestyle and temperature. Ecol Lett 13:184–193
Killen SS, Glazier DS, Rezende EL, Clark TD, Atkinson D, Willener AS, Halsey LG (2016) Ecological influences and morphological correlates of resting and maximal metabolic rates across teleost fish species. Am Nat 187:592–606
Kozłowski J, Konarzewski M, Gawelczyk AT (2003) Cell size as a link between noncoding DNA and metabolic rate scaling. Proc Natl Acad Sci USA 100:14080–14085
Kozłowski J, Czarnołęski M, Françoiskrassowska A, Maciak S, Pis T (2010) Cell size is positively correlated between different tissues in passerine birds and amphibians, but not necessarily in mammals. Biol Lett 6(6):792–796
Laughlin SB, Van Steveninck RR, Anderson J (1998) The metabolic cost of neural information. Nat Neurosci 1:36–41
Liu S, Ludwig A, Peng Z (2017) Nine novel microsatellites for the cavefish (Triplophysa rosa Chen & Yang, 2005). J Appl Ichthyol 3:119–120
Luo YP, Wang QQ (2012) Effects of body mass and temperature on routine metabolic rate of juvenile largemouth bronze gudgeon Coreius guichenoti. J Fish Biol 80:842–851
Luo YP, Wang W, Zhang Y, Huang QD (2013) Effect of body size on organ-specific mitochondrial respiration rate of the largemouth bronze gudgeon. Fish Physiol Biochem 39:513–521
Lv X, Xie H, Xia DY, Shen C, Li J, Luo YP (2018) Mass scaling of the resting and maximum metabolic rates of the black carp. J Comp Physiol B 188:591–598
Martin RD (1981) Relative brain size and basal metabolic-rate in terrestrial vertebrates. Nature 293:57–60
Mink JW, Blumenschine RJ, Adams DB (1981) Ratio of central nervous system to body metabolism in vertebrates: its constancy and functional basis. Am J Physiol 241:203–212
Moran D, Softley R, Warrant EJ (2014) Eyeless Mexican cavefish save energy by eliminating the circadian rhythm in metabolism. PLoS One 9:e107877
Moran D, Softley R, Warrant EJ (2015) The energetic cost of vision and the evolution of eyeless Mexican cavefish. Sci Adv 1:e1500363
Myagkov NA (1991) The brain sizes of living elasmobranchii as their organization level indicator. I. General analysis. J Hirnforsch 32:553–561
Nilsson G (1996) Brain and body oxygen requirements of Gnathonemus petersii, a fish with an exceptionally large brain. J Exp Biol 199:603–607
Niu Y (2017) Comparative histological study of visual organs between Triplophysa rosa and Triplophysa bleekeri. Master’s Thesis, Southwest University
Niven JE (2015) Neural evolution: costing the benefits of eye loss. Curr Biol 25:827–844
Oikawa S, Itazawa Y (1993) Tissue respiration and relative growth of parts of body of a marine teleost, porgy Pagrus major, during early life stages with special reference to the metabolism-size relationship. Comp Biochem Physiol A 105:741–744
Porter SM (2001) Effects of size and light on respiration and activity of walleye pollock (Theragra chalcogramma) larvae. J Exp Mar Biol Ecol 256:253–265
Poulson TL (2001) Morphological and physiological correlates of evolutionary reduction of metabolic rate among amblyopsid cave fishes. Environ Biol Fish 62:239–249
Poulson TL, White WB (1969) The cave environment. Science 165:971–981
Rombough PJ (1988) 2 Respiratory gas exchange, aerobic metabolism, and effects of hypoxia during early life. Fish Physiol 11:59–161
Schemmel C (1980) Studies on the genetics of feeding behavior in the cave fish Astyanax mexicanus f. Anoptichthys. An example of apparent monofactorial inheritance by polygenes. Z Tierpsychol 53(1):9–22
Schultz IR, Barron MG, Newman MC, Vick AM (1999) Blood flow distribution and tissue allometry in channel catfish. J Fish Biol 54:1275–1286
Seibel BA, Drazen JC (2007) The rate of metabolism in marine animals: environmental constraints, ecological demands and energetic opportunities. Philos Trans R Soc Lond B 362(1487):2061–2078
Soares MC, André GI, Paula JR (2015) Preliminary notes on brain weight variation across labrid fish species with different levels of cooperative behaviour. Curr Zool 61(2):274–280
Solberg T, Tilseth S (1984) Growth, energy consumption, and prey density requirements in first feeding larvae of cod (Gadus morhua L). In: Dahl E, Danielssen DP, Moksness E, Solemdal P (eds) The propagation of Cod Gadus morhua L., Part 1. Inst. Mar. Res. Flodevigen Biol. Stn., Arendal, pp. 145–166
Trajano E (2001) Ecology of subterranean fishes: an overview. Environ Biol Fish 62:133–160
Trippel EA, Neil SRE (2002) Effect of photoperiod and light intensity on growth and activity of juvenile haddock (Melanogrammus aeglefinus). Aquaculture 217:633–645
Wang J, Tang Q, Wang Z, Zhang Y, Wu Q, Peng Z (2012a) The complete mitogenome sequence of a cave loach Triplophysa rosa (Teleostei, Balitoridae, Nemacheilinae). Mitochondrial DNA 23:366–368
Wang Q, Wang W, Huang Q, Zhang YR, Luo YP (2012b) Effect of meal size on the specific dynamic action of the juvenile snakehead (Channa argus). Comp Biochem Physiol A 161:401–405
Xiao Y (2017) Genetic basis of the albinism in the cavefish Triplophysa rosa. Southwest University, Chongqing
Xie XJ, Sun RY (1990) The bioenergetics of the southern catfish (Silurus meridionalis Chen): resting metabolic rate as a function of body weight and temperature. Physiol Zool 63:1181–1195
Yu Y, Karbowski J, Sachdev RN, Feng J (2014) Effect of temperature and glia in brain size enlargement and origin of allometric body-brain size scaling in vertebrates. BMC Evol Biol 14(1):178–178
Zhang Y, Huang Q, Liu S, He DC, Wei G, Luo YP (2014) Intraspecific mass scaling of metabolic rates in grass carp (Ctenopharyngodon idellus). J Comp Physiol B 184:347–354
Zhao J, Zhao K, Peng Z (2014) Development and characterization of eleven microsatellite markers for an endangered cavefish (Triplophysa rosa Chen and Yang, 2005) using 454 sequencing. J Appl Ichthyol 30:1056–1058
Zupanc GKH (2006) Neurogenesis and neuronal regeneration in the adult fish brain. J Comp Physiol A 192:649–670
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 31672287). We thank Mr. Niu Yabing and Mr. Luo Dehuai, who made most of the equipment available to me. We thank Mr. Luo for his help in fish collection and thank the anonymous reviewers for their comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Heldmaier.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shi, C., Yao, M., Lv, X. et al. Body and organ metabolic rates of a cave fish, Triplophysa rosa: influence of light and ontogenetic variation. J Comp Physiol B 188, 947–955 (2018). https://doi.org/10.1007/s00360-018-1178-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-018-1178-x