Abstract
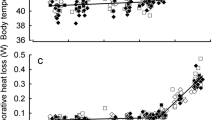
Variation in rates of water loss has been proposed to be an important mechanism in the survival of terrestrial organisms, as high rates of water loss in desiccating environments may lead to hydric stress and death. Vapor density deficit, the driving force for evaporative water loss, increases exponentially as temperature increases. Acute temperature changes may be the result of daily behavioral thermoregulation of ectotherms, which may influence the among individual variation rates of water loss. The goals of this study were to determine (1) how rates of cutaneous water loss (CWL) and skin resistance (Rs) are affected by acute temperature acclimation, (2) how rates of CWL and Rs vary throughout the day allowing behavioral thermoregulation and (3) the repeatability of CWL and Rs within and among sampling periods. We measured CWL and calculated skin resistance (Rs) of 30 male Sceloporus consobrinus lizards across three summers. We measured CWL on the dorsal and ventral surface of each lizard at 23 °C followed by measurements at 35 °C, and three separate times throughout the day. We found a significant increase in Rs and decrease in CWL at increased acclimation temperatures (35 °C), a significant difference in CWL and Rs throughout the day allowing behavioral thermoregulation, and support for the repeatability of CWL and Rs. Our results demonstrate variability in CWL and Rs in relation to temperature acclimation and thermoregulation, but mixed evidence for repeatability across treatments. Our results suggest other factors, such as peripheral blood flow, may be influencing the inter-individual variation in CWL and Rs.



Similar content being viewed by others
References
Acevedo GA (2009) Ecomorphology of the mexican fence lizards of the Sceloporus formosus group (Squamata: Phyrnosomatidae). PhD. Dissertation. The University of Texas at Arlington
Angilletta MJ, Oufiero CE, Sears MW (2004) Thermal adaptation of maternal and embryonic phenotypes in a geographically widespread ectotherm. Int Congr Ser 1275:258–266
Angilletta MJ, Oufiero CE, Leaché AD (2006) Direct and indirect effects of environmental temperature on the evolution of reproductive strategies: an information-theoretic approach. Am Nat 168:E123–35. https://doi.org/10.1086/507880
Bates DM, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48. https://doi.org/10.1177/009286150103500418
Bentley PJ, Schmidt-Nielsen K (1966) Cutaneous water loss in reptiles. Science 151:1547–1549
Boake CRB (1989) Repeatability: its role in evolutionary studies of mating behavior. Evol Ecol 3:173–182. https://doi.org/10.1007/BF02270919
Buckley LB, Urban MC, Angilletta MJ et al (2010) Can mechanism inform species’ distribution models? Ecol Lett 13:1041–1054. https://doi.org/10.1111/j.1461-0248.2010.01479.x
Burnham KP, Anderson DR (2003) Model selection and multimodel inference: a practical information-theoretic approach. Springer
Cloudsley-Thompson JL (1971) The temperature and water relations of reptiles. Merrow, Watford
Clusella-Trullas S, Terblanche JS, Van Wyk JH, Spotila JR (2007) Low repeatability of preferred body temperature in four species of Cordylid lizards: temporal variation and implications for adaptive significance. Evol Ecol 21:63–79. https://doi.org/10.1007/s10682-006-9124-x
Conradsen C, Walker JA, Perna C, McGuigan K (2016) Repeatability of locomotor performance and morphology–locomotor performance relationships. J Exp Biol 219:2888–2897. https://doi.org/10.1242/jeb.141259
Cox CL, Cox RM (2015) Evolutionary shifts in habitat aridity predict evaporative water loss across squamate reptiles. Evolution 69:2507–2516
Crowley SR (1987) The effect of desiccation upon the preferred body temperature and activity level of the lizard Sceloporus undulatus. Copeia 25–32
Deutsch CA, Tewksbury JJ, Huey RB et al (2008) Impacts of climate warming on terrestrial ectotherms across latitude. Proc Natl Acad Sci 105:6668–6672
Dmi’el R (1972) Effect of activity and temperature on metabolism and water loss in snakes. Am J Physiol Content 223:510–516
Dmi’el R (1985) Effect of body size and temperature on skin resistance to water loss in a desert snake. J Therm Biol 10:145–149
Dmi’el R (1998) Skin resistance to evaporative water loss in viperid snakes: habitat aridity versus taxonomic status. Comp Biochem Physiol Part A Mol Integr Physiol 121:1–5
Dml’el R, Perry G, Lazell J (1997) Evaporative water loss in nine insular populations of the lizard Anolis cristatellus group in the British Virgin Islands. Biotropica 29:111–116
Dohm MR (2002) Repeatability estimates do not always set an upper limit to heritability. Funct Ecol 16:273–280
Eynan M, Dmi’el R (1993) Skin resistance to water loss in agamid lizards. Oecologia 95:290–294
Gilbert AL, Miles DB (2016) Food, temperature and endurance: effects of food deprivation on the thermal sensitivity of physiological performance. Funct Ecol 30:1790–1799
Hayes JP, Bible CA, Boone JD (1998) Repeatability of mammalian physiology : evaporative water loss and oxygen consumption of Dipodomys merriami. J Mammal 79:475–485
Kobayashi D, Mautz WJ, Nagy KA (1983) Evaporative water loss: humidity acclimation in Anolis carolinensis lizards. Copeia 701–704
Kuznetsova A, Brockhoff PB, Christensen RHB (2015) Package ‘lmerTest’. R package version 2(0)
Leaché AD, Reeder TW (2002) Molecular systematics of the eastern fence lizard (Sceloporus undulatus): a comparison of parsimony, likelihood, and Bayesian approaches. Syst Biol 51:44–68
Lillywhite HB (2006) Water relations of tetrapod integument. J Exp Biol 209:202–226. https://doi.org/10.1242/jeb.02007
Mautz WJ (1980) Factors influencing evaporative water loss in lizards. Comp Biochem Physiol Part A Physiol 67:429–437
Mautz WJ (1982) Correlation of both respiratory and cutaneous water losses of lizards with habitat aridity. J Comp Physiol 149:25–30
Moreshet S (1970) Effect of environmental factors on cuticular transpiration resistance. Plant Physiol 46:815–818
Muñoz-Garcia A, Ro J, Reichard JD et al (2012) Cutaneous water loss and lipids of the stratum corneum in two syntopic species of bats. Comp Biochem Physiol A Mol Integr Physiol 161:208–215. https://doi.org/10.1016/j.cbpa.2011.10.025
Oufiero CE, Angilletta MJ Jr (2006) Convergent evolution of embryonic growth and development in the eastern fence lizard (Sceloporus undulatus). Evolution 60:1066–1075
Oufiero CE, Garland T (2009) Repeatability and correlation of swimming performances and size over varying time scales in the guppy (Poecilia reticulata). Funct Ecol 23:969–978
Oufiero CE, Gartner GEA, Adolph SC, Garland T Jr (2011) Latitudinal variation in scale counts and body size in Sceloporus lizards: a phylogenetic perspective. Evolution 65:3590–3607
Perry G, Dmi’el R, Lazell J (1999) Evaporative water loss in insular populations of the Anolis cristatellus group (Reptilia: Sauria) in the British Virgin Islands II: the effects of drought. Biotropica 31(2):337–343
Perry G, Dmi’el R, Lazell J (2000) Evaporative water loss in insular populations of Anolis cristatellus (Reptilia: Sauria) in the British Virgin Islands. III. Response to the end of drought and a common garden experiment. Biotropica 32:722–728
Prange HD, Schmidt-Nielsen K (1969) Evaporative water loss in snakes. Comp Biochem Physiol 28:973–975
Riddell EA, Sears MW (2015) Geographic variation of resistance to water loss within two species of lungless salamanders: implications for activity. Ecosphere 6:art86. https://doi.org/10.1890/ES14-00360.1
Sinervo B, Mendez-De-La-Cruz F, Miles DB et al (2010) Erosion of lizard diversity by climate change and altered thermal niches. Science 328:894–899
Tingley R, Greenlees MJ, Shine R (2012) Hydric balance and locomotor performance of an anuran (Rhinella marina) invading the Australian arid zone. Oikos 121:1959–1965. https://doi.org/10.1111/j.1600-0706.2012.20422.x
Tu MC, Lillywhite HB, Menon JG, Menon GK (2002) Postnatal ecdysis establishes the permeability barrier in snake skin: new insights into barrier lipid structures. J Exp Biol 205:3019–3030
Van Sant MJ, Oufiero CE, Muñoz-Garcia A et al (2012) A phylogenetic approach to total evaporative water loss in mammals. Physiol Biochem Zool 85:526–532. https://doi.org/10.1086/667579
Versteegh M, Helm B, Dingemanse NJ, Tieleman BI (2008) Repeatability and individual correlates of basal metabolic rate and total evaporative water loss in birds: a case study in European stonechats. Comp Biochem Physiol A Mol Integr Physiol 150:452–457. https://doi.org/10.1016/j.cbpa.2008.05.006
Williams JB (1996) A phylogenetic perspective of evaporative water loss in birds. Auk 457–472
Wolak ME, Fairbairn DJ, Paulsen YR (2012) Guidelines for estimating repeatability. Methods Ecol Evol 3:129–137. https://doi.org/10.1111/j.2041-210X.2011.00125.x
Acknowledgements
We thank the agencies at the Oklahoma Department of Wildlife Conservation and the Wichita Mountains Wildlife Refuge for permits to collect specimens. We also thank Rhagan Hill for help catching lizards and two anonymous reviewers for comments that improved the clarity of the manuscript. The research was funded by a Towson University Faculty Development and Research Committee Award to C.E.O.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by I. D. Hume.
Rights and permissions
About this article
Cite this article
Oufiero, C.E., Van Sant, M.J. Variation and repeatability of cutaneous water loss and skin resistance in relation to temperature and diel variation in the lizard Sceloporus consobrinus. J Comp Physiol B 188, 671–681 (2018). https://doi.org/10.1007/s00360-018-1156-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-018-1156-3