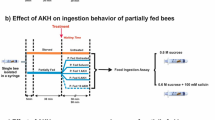
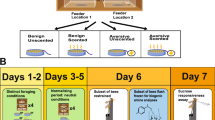
Abstract
Insects have rapidly changing energy demands, so they primarily rely on hemolymph and other carbohydrates to carry out life activities. However, how gustatory responsiveness and hemolymph sugar levels coordinate with one another to maintain energetic homeostasis in insects remains largely unknown for the highly social honeybee that goes through large physiological and behavioral changes. The potential role of biogenic amines and neuropeptides in the connection between the regulation of appetite and fluctuating sugar levels in the hemolymph, due to starvation, as the bee ages, was investigated. The largest appetite increase due to the starvation treatment was within the forager age class and this corresponded with an increase in octopamine levels in the brain along with a decline in hemolymph sugar levels. Adipokinetic hormone (AKH) was found in very small quantities in the brain and there were no significant changes in response to starvation treatment. Our findings suggest that the particularly dynamic levels of hemolymph sugar levels may serve as a monitor of the forager honeybee energetic state. Therefore, there may be a pathway in forager bees via octopamine responsible for their precise precipitous regulation of appetite, but to determine cause and effect relationships further investigation is needed.




Similar content being viewed by others
Abbreviations
- AKH:
-
Adipokinetic hormone
- GC–MS:
-
Gas chromatography–mass spectrometry
- GLM:
-
Generalized linear model
- GRS:
-
Gustatory response score
- HPLC:
-
High-performance liquid chromatography
- MANOVA:
-
Multivariate analysis of variance
- PER assay:
-
Proboscis extension response assay
References
Amdam GV, Omholt SW (2003) The hive bee to forager transition in honeybee colonies: the double repressor hypothesis. J Theor Biol 223:451–464
Amdam GV, Norberg K, Page RE Jr, Erber J, Scheiner R (2006) Downregulation of vitellogenin gene activity increases the gustatory responsiveness of honey bee workers (Apis mellifera). Behav Brain Res 169:201–205
Behrends A, Scheiner R, Baker N, Amdam GV (2007) Cognitive aging is linked to social role in honey bees (Apis mellifera). Exp Gerontol 42(12):1146–1153
Bitterman ME, Menzel R, Fietz A, Schäfer S (1983) Classical conditioning of proboscis extension in honeybees (Apis mellifera). J Comp Psychol 97:107–119
Blatt J, Roces F (2001) Haemolymph sugar levels in foraging honeybees (Apis mellifera carnica): dependence on metabolic rate and in vivo measurement of maximal rates of trehalose synthesis. J Exp Biol 204:2709–2716
Blatt J, Roces F (2002) The control of proventriculus in the honeybee (Apis mellifera carnica L.) I. A dynamic process influenced by food quality and quantity? J Insect Physiol 48:643–654
Bounias M (1987) Propranolol and somatostatin interactions with the hyperglycemic effects of glucagon, octopamine, noradrenaline and cAMP in worker honeybees in vivo. Biochimie 69(6–7):655–660
Bounias M, Kruk I (1983) Adrenochrome as competitive inhibitor of epinephrine effects: a comparative study on trehalose and glucose levels in the honey-bee hemolymph. Comp Biochem Physiol C 74(1):143–150. https://doi.org/10.1016/0742-8413(83)90166-4
Brockmann A, Annangudi SP, Richmond TA, Ament SA, Xie F, Southey BR, Rodriguez-Zas SR, Robinson GE, Sweedler JV (2009) Quantitative peptidomics reveal brain peptide signatures of behavior. Proc Natl Acad Sci 106(7):2383–2388. https://doi.org/10.1073/pnas.0813021106
Candy DJ (1978) Regulation of locust flight-muscle metabolism by octopamine and other compounds. Insect Biochem 8(3):177–181. https://doi.org/10.1016/0020-1790(78)90070-7
Chan Q, Mutti NS, Foster LJ, Kocher SD, Amdam GV, Wolschin F (2011) The worker honeybee fat body proteome is extensively remodeled preceding a major life-history transition. PLoS One 6(9):e24794. https://doi.org/10.1371/journal.pone.0024794
Cohen RW, Friedman S, Waldbauer GP (1988) Physiological control of nutrient self-selection in Heliothis zea larvae: the role of serotonin. J Insect Physiol 34(10):935–940. https://doi.org/10.1016/0022-1910(88)90129-1
Cohen R, Mahoney D, Can H (2002) Possible regulation of feeding behavior in cockroach nymphs by the neurotransmitter octopamine. J Insect Behav 15(1):37–50
Crailsheim K (1988) Intestinal transport of sugars in the honeybee (Apis mellifera L.). J Insect Physiol 34:839–845
Davenport AP, Evans PD (1984) Changes in hemolymph octopamine levels associated with food-deprivation in the locust Schistocerca-gregaria. Physiol Entomol 9(3):269–274
Evans JD, Schwarz RS, Chen YP, Budge G, Cornman RS, Rua P, Miranda J, Foret S, Foster L, Gauthier L, Genersch E, Gisder S, Jarosch A, Kucharski R, Lopez D, Lun CM, Moritz RFA, Maleszka R, Muñoz I, Pinto MA (2013) Coloss beebook volume I: standard methods for Apis mellifera research, 1st edn. Ibra Publications, Bristol, p 636
Fell RD (1990) The qualitative and quantitative analysis of insect hemolymph sugars by high-performance thin-layer chromatography. Comp Biochem Physiol 95(4):539–544
Fields PE, Woodring JP (1991) Octopamine mobilization of lipids and carbohydrates in the house cricket, Acheta domesticus. J Insect Physiol 37(3):193–199. https://doi.org/10.1016/0022-1910(91)90069-C
Friedman S, Waldbauer GP, Eertmoed JE, Naeem M, Ghent AW (1991) Blood trehalose levels have a role in the control of dietary self-selection by Heliothis zea larvae. J Insect Physiol 37(12):919–928. https://doi.org/10.1016/0022-1910(91)90007-m
Fryer JH, Moore NS, Williams HH, Young CM (1955) A study of the interrelationship of the energy-yielding nutrients, blood glucose levels, and subjective appetite in man. J Lab Clin Med 45(5):684–696
Harris JW, Woodring J (1992) Effects of stress, age, season, and source colony on levels of octopamine, dopamine and serotonin in the honey bee (Apis mellifera L.) brain. J Insect Physiol 38(1):29–35
Harrison JM (1986) Caste-specific changes in honeybee flight capacity. Physiol Zool 59(2):175–187
Ishida Y, Ozaki M (2011) A putative octopamine/tyramine receptor mediating appetite in a hungry fly. Naturwissenschaften 98(7):635–638. https://doi.org/10.1007/s00114-011-0806-z
Jaycox ER, Skowrone W, Guynn G (1974) Behavioral-changes in worker honey bees (Apis mellifera) induced by injections of a juvenile hormone mimic. Ann Entomol Soc Am 67(4):529–535
Klowden M (2013) Physiological systems in insects, 3rd edn. Academic Press, New York, p 677
Kodrík D (2008) Adipokinetic hormone functions that are not associated with insect flight. Physiol Entomol 33(3):171–180. https://doi.org/10.1111/j.1365-3032.2008.00625.x
Konuma T, Morooka N, Nagasawa H, Nagata S (2012) Knockdown of the adipokinetic hormone receptor increases feeding frequency in the two-spotted cricket Gryllus bimaculatus. Endocrinology 153(7):3111–3122
Koon AC, Ashley J, Barria R, DasGupta S, Brain R, Waddell S, Alkema MJ, Budnik V (2011) Autoregulatory and paracrine control of synaptic and behavioral plasticity by octopaminergic signaling. Nat Neurosci 14(2):190–199. https://doi.org/10.1038/nn.2716
Lange AB (2009) Tyramine: from octopamine precursor to neuroactive chemical in insects. Gen Comp Endocrinol 162(1):18–26. https://doi.org/10.1016/j.ygcen.2008.05.021
Lee GH, Park JH (2004) Hemolymph sugar homeostasis and starvation-induced hyperactivity affected by genetic manipulations of the adipokinetic hormone-encoding gene in Drosophila melanogaster. Genetics 167(1):311–323. https://doi.org/10.1534/genetics.167.1.311
Leibowitz S, Stanley B (1986) Neurochemical controls of appetite. In: Rolls E (ed) Feeding behavior: neural and humoral controls. Academic Press, New York, pp 191–234
Linn CE, Roelofs WL (1993) Levels of biogenic amines and peptides in individual corn earworm moths, Helicoverpa zea, using high performance liquid chromatography with electrochemical detection. Insect Biochem Mol Biol 23(3):367–373. https://doi.org/10.1016/0965-1748(93)90020-S
Long TF, Murdock LL (1983) Stimulation of blowfly feeding-behavior by octopaminergic drugs. Proc Natl Acad Sci Biol 80(13):4159–4163. https://doi.org/10.1073/pnas.80.13.4159
Lorenz MW, Kellner R, Woodring J, Hoffmann KH, Gade G (1999) Hypertrehalosaemic peptides in the honeybee (Apis mellifera): purification, identification and function. J Insect Physiol 45(7):647–653
Lorenz MW, Kellner R, Völkl W, Hoffmann KH, Woodring J (2001) A comparative study on hypertrehalosaemic hormones in the Hymenoptera: sequence determination, physiological actions and biological significance. J Insect Physiol 47(6):563–571
Mayack C, Naug D (2010) Parasitic infection leads to decline in hemolymph sugar levels in honeybee foragers. J Insect Physiol 56(11):1572–1575
Mayack C, Naug D (2011) A changing but not an absolute energy budget dictates risk-sensitive behaviour in the honeybee. Anim Behav 82(3):595–600
Mayack C, Naug D (2013) Individual energetic state can prevail over social regulation of foraging in honeybees. Behav Ecol Sociobiol 67(6):929–936. https://doi.org/10.1007/s00265-013-1517-6
Medeiros PM, Simoneit BRT (2007) Gas chromatography coupled to mass spectrometry for analyses of organic compounds and biomarkers as tracers for geological, environmental, and forensic research. J Sep Sci 30(10):1516–1536. https://doi.org/10.1002/jssc.200600399
Nisimura T, Seto A, Nakamura K, Miyama M, Nagao T, Tamotsu S, Yamaoka R, Ozaki M (2005) Experiential effects of appetitive and nonappetitive odors on feeding behavior in the blowfly, Phormia regina: a putative role for tyramine in appetite regulation. J Neurosci 25:7507–7516
Pankiw T, Page RE Jr (1999) The effect of genotype, age, sex, and caste on response thresholds to sucrose and foraging behavior of honey bees (Apis mellifera L.). J Comp Physiol A 185:207–213
Pankiw T, Page RE Jr (2000) Response thresholds to sucrose predict foraging division of labor in honeybees. Behav Ecol Sociobiol 47:265–267
Pankiw T, Nelson M, Page RE, Fondrk MK (2004) The communal crop: modulation of sucrose response thresholds of pre-foraging honey bees with incoming nectar quality. Behav Ecol Sociobiol 55:286–292
Robinson GE (1985) Effects of a juvenile hormone analogue on honey bee foraging behaviour and alarm pheromone production. J Insect Physiol 31:277–282
Robinson GE (1987) Regulation of honey bee age polyethism by juvenile hormone. Behav Ecol Sociobiol 20:329–338
Roeder T, Seifert M, Kahler C, Gewecke M (2003) Tyramine and octopamine: antagonistic modulators of behavior and metabolism. Arch Insect Biochem Physiol 54(1):1–13. https://doi.org/10.1002/arch.10102
Scheiner R (2006) Aminergic control and modulation of honeybee behaviour. Curr Neuropharmacol 4:259–276
Scheiner R, Plückhahn S, Öney B, Blenau W, Erber J (2002) Behavioural pharmacology of octopamine, tyramine and dopamine in honey bees. Behav Brain Res 136(2):545–553
Scheiner R, Page RE, Erber J (2004) Sucrose responsiveness and behavioral plasticity in honey bees (Apis mellifera). Apidologie 35:133–142
Schulz DJ, Robinson GE (1999) Biogenic amines and division of labor in honey bee colonies: behaviorally related changes in the antennal lobes and age-related changes in the mushroom bodies. J Comp Physiol A 184(5):481–488. https://doi.org/10.1007/s003590050348
Schulz DJ, Robinson GE (2001) Octopamine influences division of labor in honey bee colonies. J Comp Physiol A 187(1):53–61
Schulz DJ, Barron AB, Robinson GE (2002a) A role for octopamine in honey bee division of labor. Brain Behav Evolut 60(6):350–359
Schulz DJ, Sullivan JP, Robinson GE (2002b) Juvenile hormone and octopamine in the regulation of division of labor in honey bee colonies. Horm Behav 42(2):222–231. https://doi.org/10.1006/hbeh.2002.1806
Seeley TD (1982) Adaptive significance of the age polyethism schedule in honeybee colonies. Behav Ecol Sociobiol 11:287–293
Simcock NK, Gray H, Bouchebti S, Wright GA (2018) Appetitive olfactory learning and memory in the honeybee depend on sugar reward identity. J Insect Physiol 106(Pt 1):71–77. https://doi.org/10.1016/j.jinsphys.2017.08.009
Simpson SJ, Raubenheimer D (1993) The central role of the haemolymph in the regulation of nutrient intake in insects. Physiol Entomol 18:395–403
Thompson SN (2003) Trehalose—the insect ‘blood’ sugar. Adv Insect Physiol 31:205–285
Toth AL, Robinson GE (2005) Worker nutrition and division of labour in honeybees. Anim Behav 69(2):427–435
Toth AL, Kantarovich S, Meisel AF, Robinson GE (2005) Nutritional status influences socially regulated foraging ontogeny in honey bees. J Exp Biol 208:4641–4649
Van der Horst DJ (2003) Insect adipokinetic hormones: release and integration of flight energy metabolism. Comp Biochem Physiol B 136(2):217–226. https://doi.org/10.1016/S1096-4959(03)00151-9
Veenstra JA, Rodriguez L, Weaver RJ (2012) Allatotropin, leucokinin and AKH in honey bees and other hymenoptera. Peptides 35(1):122–130
Wada-Katsumata A, Yamaoka R, Aonuma H (2011) Social interactions influence dopamine and octopamine homeostasis in the brain of the ant Formica japonica. J Exp Biol 214(10):1707–1713. https://doi.org/10.1242/jeb.051565
Wagener-Hulme C, Kuehn JC, Schulz DJ, Robinson GE (1999) Biogenic amines and division of labor in honey bee colonies. J Comp Physiol A 184(5):471–479. https://doi.org/10.1007/s003590050347
Wang Y, Brent CS, Fennern E, Amdam GV (2012) Gustatory perception and fat body energy metabolism are jointly affected by vitellogenin and juvenile hormone in honey bees. PLoS Genet 8(6):e1002779. https://doi.org/10.1371/journal.pgen.1002779
Woodring J, Boulden M, Das S, Gäde G (1993) Studies on blood sugar homeostasis in the honeybee (Apis mellifera, L.). J Insect Physiol 39(1):89–97
Woodring J, Das S, Gäde G (1994) Hypertrehalosemic factors from the corpora cardiaca of the honeybee (Apis mellifera) and the paper wasp (Polistes exclamans). J Insect Physiol 40(8):685–692
Acknowledgements
We thank Mariann Pü, Brian Shields, Rebecca Zhou, Victor Le, and Elizabeth Eppley for assistance with hemolymph extractions, brain dissections, and data collection. The authors would also like to thank Dr. Kathleen Howard for providing access to her lyophilizer and assisting with its use. This work was supported by an Alexander von Humboldt Foundation Fellowship award to CM, a Swarthmore College Surdna Summer Research Fellowship to KC, and a Howard Hughes Medical Institute Fellowship to NP.
Data availability
The datasets generated during the current study are available in the Open Science Framework repository, https://osf.io/h4c6e/.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All honeybees were handled humanely in accordance with current ethical standards. Special ethical approval is not required to carry out this study.
Conflict of interest
The authors declare that there are no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mayack, C., Phalen, N., Carmichael, K. et al. Appetite is correlated with octopamine and hemolymph sugar levels in forager honeybees. J Comp Physiol A 205, 609–617 (2019). https://doi.org/10.1007/s00359-019-01352-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-019-01352-2