Abstract
Purpose
In 2003, the German Cancer Society (Deutsche Krebsgesellschaft, DKG) launched a certification program aimed at improving the quality of cancer care. The purpose of this article is to describe the experience of the Prostate Cancer Unit (PCU) at Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, in the process towards DKG certification.
Methods
In 2018, PCU decided to apply for certification by adopting DKG catalogue of requirements (CoR) and quality indicators. A multiprofessional working group was established with the aim of acting the necessary steps to meet DKG standards.
Results
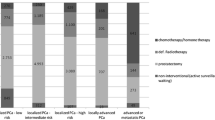
Our organizational setting (procedures, personnel) and activities were accurately analyzed, thus outlining strengths and weaknesses, and modified to comply with DKG CoR and indicators. As examples, (1) a quality management plan was developed; (2) measures were taken to strengthen the surgical expertise; (3) cases evaluated in weekly tumor boards were expanded to include surgical cases with pathological risk factors, metastatic, relapsed and castration-resistant patients; (4) a survey was added to the patient-dedicated initiatives already scheduled; (5) the TuDoc software became the tool to register all new cases of prostate cancer patients referred to PCU.
Conclusions
The process of certification requires many efforts but represents a unique opportunity of improving quality of care of prostate cancer patients, making it comparable on an international scale.
Similar content being viewed by others
References
European Partnership Action Against Cancer consensus group (2014) Policy statement on multidisciplinary cancer care. Eur J Cancer 50:475–480
Fennell ML, Das IP, Clauser S et al (2010) The organization of multidisciplinary care teams: modeling internal and external influences on cancer care quality. J Natl Cancer Inst Monogr 40:72–80
Kurpad R, Kim W, Rathmell WK et al (2011) A multidisciplinary approach to the management of urologic malignancies: does it influence diagnostic and treatment decisions? Urol Oncol 29:378–382
Kesson EM, Allardice GM, George WD et al (2012) Effects of multidisciplinary team working on breast cancer survival: retrospective, comparative, interventional cohort study of 13722 women. BMJ 344:e2718
Jelenc M, Van Hoof E, Albreht T et al (2012) Joint action European partnership for action against cancer. Arch. Public Health 70:24
Magnani T, Bracarda S, D'Angelillo RM et al (2019) Multidisciplinary teams for the proper management of patients with genitourinary tumors: When topics set scientific societies' agenda. Tumori 105:161–167
Valdagni R, Albers P, Bangma C et al (2011) The requirements of a specialist prostate cancer unit: a discussion paper from the European School of Oncology. Eur J Cancer 47:1–7
Valdagni R, Van Poppel H, Aitchison M et al (2015) Prostate Cancer Unit Initiative in Europe: a position paper by the European School of Oncology. Crit Rev Oncol Hematol 95:133–143
Wirth M, Fossati N, Albers P et al (2019) The European Prostate Cancer Centres of Excellence: A Novel Proposal from the European Association of Urology Prostate Cancer Centre Consensus Meeting. Eur Urol. https://doi.org/10.1016/j.eururo.2019.01.033
NICE Guidance (2019) NICE guidelines prostate cancer. BJU Int. 124:9–26
National Comprehensive Cancer Network. Prostate Cancer (Version 2.2019) (2019) https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf
Mottet N, Bellmunt J, Bolla M et al (2017) EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol 71:618–629
Wolff RF, Ryder S, Bossi A et al (2015) A systematic review of randomised controlled trials of radiotherapy for localised prostate cancer. Eur J Cancer 51:2345–2367
Potosky AL, Davis WW, Hoffman RM et al (2004) Five-year outcomes after prostatectomy or radiotherapy for prostate cancer: the prostate cancer outcomes study. J Natl Cancer Inst 96:1358–1367
Hamdy FC, Donovan JL, Lane JA et al (2016) 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med 375:1415–1424
Sanda MG, Dunn RL, Michalski J et al (2008) Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med 358:1250–1261
Donovan JL, Hamdy FC, Lane JA et al (2016) Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med 375:1425–1437
Bray F, Ferlay J, Soerjomataram I et al (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424
Wesselman S, Beckmann MW (2014) The concept of the certification system of the German Cancer Society and its impact on gynecological cancer care. Arch Gynecol Obstet 289:7–12
Kowalski C, Graeven U, von Kalle C et al (2017) Shifting cancer care towards multidisciplinarity: the cancer center certification program of the German cancer society. BMC Cancer 17:850–859
Saghatchian M, Thonon F, Boomsma F et al (2014) Pioneering quality assessment in European cancer centers: a data analysis of the organization for European cancer institutes accreditation and designation program. J Oncol Pract 10:e342–e349
Saghatchian M, Hummel H, Otter R et al (2008) Organisation of European Cancer Institutes. Towards quality, comprehensiveness and excellence. The accreditation project of the Organisation of European Cancer Institutes (OECI). Tumori 94:164–171
Griesshammer E, Wesselmann S (2019) European Cancer Centre Certification Programme Der Gynäkologe A European way to quality of cancer care. https://doi.org/10.1007/s00129-019-4398-6
Magnani T, Valdagni R, Salvioni R et al (2012) The 6-year attendance of a multidisciplinary prostate cancer clinic in Italy: incidence of management changes. BJU Int 110:998–1003
Voigt W, Hoellthaler J, Magnani T et al (2014) 'Act on oncology' as a new comprehensive approach to assess prostate cancer centres—method description and results of a pilot study. PLoS ONE 9:e106743. https://doi.org/10.1371/journal.pone.0106743
Spencer BA, Miller DC, Litwin MS et al (2008) Variations in quality of care for men with early-stage prostate cancer. J Clin Oncol 26:3735–3742
Center MM, Jemal A, Lortet-Tieulent J et al (2012) International variation in prostate cancer incidence and mortality rates. Eur Urol 61:1079–1092
Acknowledgements
The authors wish to thank Fondazione Italo Monzino, Milan, for the support to the activities of the Prostate Cancer Program and the Prostate Cancer Unit. A special thank to the Prostate Cancer Unit Core Team, Non Core Team and Project Team who participate in the management of prostate cancer patients: A. Alessi, B. Avuzzi, F. Badenchini, A. Balzarini, L. Bellardita, C. Borreani, A. Candosin, M. Carrara, A. Casale, M. Catanzaro, A. Cavallo, A. Cerrotta, M. Claps, M. Colecchia, L. De Luca, T. Di Florio, S. Donegani, P. Dordoni, C. Fallai, L. Gatto, T. Giandini, R. Lanocita, M. Maccauro, A. Macchi, T. Magnani, C. Marenghi, C. Martini, A. Messina, S. Morlino, N. Nicolai, B. Noris Chiorda, E. Pignoli, G. Procopio, C. Ripamonti, E. Seregni, C. Spreafico, S. Stagni, A. Tesone, T. Torelli, M. Vaiani, R. Valdagni, E. Verzoni, S. Villa, F. Zollo
Author information
Authors and Affiliations
Contributions
BNC: Manuscript concept, Manuscript writing. FZ: Data collection, Manuscript concept, Manuscript writing. TM: Coordination, Manuscript concept, Manuscript writing. FB: Data collection, Manuscript review. MC: Manuscript review. LG: Data collection, Manuscript review. AM: Manuscript review. LA: Manuscript review. NN: Manuscript review. SV: Manuscript concept, Manuscript review. RV: Manuscript concept, Manuscript review.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest and no grant supported the research described in the paper.
Research involving human or animal participants
This article does not contain any studies with human participants or animals performed by any of the authors and there was no need to collect an informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Noris Chiorda, B., Zollo, F., Magnani, T. et al. How to implement the requirements of a quality assurance system for prostate cancer. World J Urol 39, 41–47 (2021). https://doi.org/10.1007/s00345-019-03024-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-019-03024-x