Abstract
Introduction and objectives
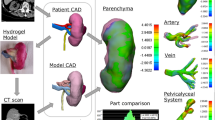
There is a scarcity of high-fidelity, life-like, standardized and anatomically correct polymer-based kidney models for robot-assisted partial nephrectomy (RAPN) simulation training. The purpose of this technical report is to present mechanical and functional testing data as evidence for utilizing a perfused hydrogel kidney model created utilizing 3D printed injection casts for RAPN simulation and training.
Methods
Anatomically correct, tumor-laden kidney models were created from 3D-printed casts designed from a patient's CT scan and injected with poly-vinyl alcohol (PVA). A variety of testing methods quantified Young’s modulus in addition to comparing the functional effects of bleeding and suturing among fresh porcine kidneys and various formulations of PVA kidneys.
Results
7% PVA at three freeze–thaw cycles (7%-3FT) was found to be the formula that best replicates the mechanical properties of fresh porcine kidney tissue, where mean(± SD) values of Young’s modulus of porcine tissue vs 7%-3FT samples were calculated to be 85.97(± 35) kPa vs 80.97(± 9.05) kPa, 15.7(± 1.6) kPa vs 74.56(± 10) kPa and 87.46(± 2.97) kPa vs 83.4(± 0.7) kPa for unconfined compression, indentation and elastography testing, respectively. No significant difference was seen in mean suture tension during renorrhaphy necessary to achieve observable hemostasis and capsular violation during a simulated perfusion at 120 mmHg.
Conclusions
This is the first study to utilize extensive material testing analyses to determine the mechanical and functional properties of a perfused, inanimate simulation platform for RAPN, fabricated using a combination of image segmentation, 3D printing and PVA casting.







Similar content being viewed by others
Change history
20 January 2020
The Eqs. 1, 2 and 3 come under the section “Kidney cortex testing” as per the original manuscript, but they have been incorrectly moved and separated into different sections in the original publication of the article.
References
Gilbody J, Prasthofer AW, Ho K et al (2011) The use and effectiveness of cadaveric workshops in higher surgical training: a systematic review. Ann R Coll Surg Engl 93:347–352
Ross HM, Simmang CL, Fleshman JW, Marcello PW (2008) Adoption of laparoscopic colectomy: results and implications of ASCRS hands-on course participation. Surg Innov 15:179–183
Van Bruwaene S, Schijven MP, Napolitano D, De Win G, Miserez M (2015) Porcine cadaver organ or virtual-reality simulation training for laparoscopic cholecystectomy: a randomized, controlled trial. J Surg Educ. 72(3):483–490
LeBlanc F, Champagne BJ, Augestad KM et al (2010) A comparison of human cadaver and augmented reality simulator models for straight laparoscopic colorectal skills acquisition training. J Am Coll Surg 211:250–255
Stefanidis D, Yonce TC, Green JM, Coker AP (2013) Cadavers versus pigs: which are better for procedural training of surgery residents outside the OR? Surgery 154(1):34–37. https://doi.org/10.1016/j.surg.2013
Villegas L, Schneider BE, Callery MP, Jones D (2003) Laparoscopic skills training. Surg Endosc 17(12):1879–1888
Candela B, Stone JJ, Park J, Guan W, Rashid H, Joseph J, Ghazi A (2016) Concurrent validity of a simulated inanimate model for physical learning experience in partial nephrectomy (SIMPLE-PN). J Urol 195(4S Supplement):e220
Ghazi A, Stone JJ, Candela B, Richards M, Joseph J (2015) Simulated inanimate model for physical learning experience (SIMPLE) for robotic partial nephrectomy using a 3-D printed kidney model. J Urol 193(4S):e778
Li P, Jiang S, Yu Y, Yang J, Yang Z (2015) Biomaterial characteristics and application of silicone rubber and PVA hydrogels mimicked in organ groups for prostate brachytherapy. J Mech Behav Biomed Mater 49:220–234. https://doi.org/10.1016/j.jmbbm.2015.05.012
Umale S, Deck C, Bourdet N et al (2013) Experimental mechanical characterization of abdominal organs: liver, kidney & spleen. J Mech Behav Biomed Mater 17:22–33
Miller K (2005) Method of testing very soft biological tissues in compression. J Biomech 38(1):153–158
Maas SA, Ellis BJ, Ateshian GA, Weiss JA (2012) FEBio: finite elements for biomechanics. J Biomech Eng 134(1):011005
Boës S, Ochsner G, Amacher R, Petrou A, Meboldt M, Schmid Daners M (2018) Control of the fluid viscosity in a mock circulation. Artif Organs 42:68–77. https://doi.org/10.1111/aor.12948
Endres DM, Bossemeyer RW, Tobert CM, Baer WH, Lane BR (2014) Investigation of forces involved in closure of the renal remnant after simulated partial nephrectomy. Urology 84(4):971–975
Benway BM, Wang AJ, Cabello JM et al (2009) Robotic partial ne- phrectomy with sliding-clip renorrhaphy: technique and outcomes. Eur Urol 55:592–599
Bartellas M (2016) Three-dimensional printing and medical education: a narrative review of the literature. UOJM. 6:1–38. https://doi.org/10.18192/uojm.v6i1.1515
Yang B, Zeng Q, Yinghao S et al (2009) A novel training model for laparoscopic partial nephrectomy using porcine kidney. J Endourol 23:2029–2033
Silberstein JL, Maddox MM, Dorsey P, Feibus A, Thomas R, Lee BR (2014) Physical models of renal malignancies using stand- ard cross-sectional imaging and 3-dimensional printers: a pilot study. Urology 84(2):268–272. https://doi.org/10.1016/j.urology.2014.03.042
Knoedler M, Feibus AH, Lange A, Maddox MM, Ledet E, Thomas R (2015) Silberstein JL Individualized physical 3-dimen- sional kidney tumor models constructed from 3-dimensional print- ers result in improved trainee anatomic understanding. Urology 85(6):1257–1261. https://doi.org/10.1016/j.urology.2015.02.053
Zhang Y, Ge HW, Li NC, Yu CF, Guo HF, Jin SH, Liu JS, Na YQ (2016) Evaluation of three-dimensional printing for laparoscopic partial nephrectomy of renal tumors: a preliminary report. World J Urol 34(4):533–537. https://doi.org/10.1007/s00345-015-1530-7
Shin T, Ukimura O, Gill IS (2016) Three-dimensional printed model of prostate anatomy and targeted biopsy-proven index tumor to facilitate nerve-sparing prostatectomy. Eur Urol 69(2):377–379. https://doi.org/10.1016/j.eururo.2015.09.024
Farshad M, Barbezat M, Flueler P, Schmidlin F et al (1999) Material characterization of the pig kidney in relation with the biomechanical analysis of renal trauma. J Biomech 32:417–425
Canales BK, Lynch AC, Fernandes E et al (2007) Novel technique of knotless hemostatic renal parenchymal suture repair during laparoscopic partial nephrectomy. Urology 70:358–359
Patel MN, Menon M, Rogers CG (2010) Robotic partial nephrectomy: a comparison to current techniques. Urol Oncol 28:74–77
Benway BM, Wang AJ, Cabello JM et al (2009) Robotic partial nephrectomy with sliding-clip renorrhaphy: technique and outcomes. Eur Urol 55:592–599
Qiu K, Zhao Z, Haghiashtiani G et al (2018) 3D printed organ models with physical properties of tissue and integrated sensors. Adv Mater Technol 3(3):170–235
Von Rundstedt FC, Scovell JM, Agrawal S et al (2017) Utility of patient-specific silicone renal models for planning and rehearsal of complex tumour resections prior to robot-assisted laparoscopic partial nephrectomy. BJU Int 119(4):598–604
Maddox MM, Feibus A, Liu J et al (2018) 3D-printed soft-tissue physical models of renal malignancies for individualized surgical simulation: a feasibility study. J Robot Surg 12(1):27–33
Carey JN, Minneti M, Leland HA et al (2015) Perfused fresh cadavers: method for application to surgical simulation. Am J Surg 210:179–187
Faure JP, Breque C, Danion J, Delpech PO, Oriot D, Richer JP (2017) SIM Life: a new surgical simulation device using a human perfused cadaver. Surg Radiol Anat 39:211–217
Udomsawaengsup S, Pattana-arun J, Tansatit T et al (2005) Minimally invasive surgery training in soft cadaver (MIST-SC). J Med Assoc Thai 88:189–194
Giger U, Fresard I, Hafliger A, Bergmann M, Krähenbühl L (2008) Laparoscopic training on Thiel human cadavers: a model to teach advanced laparoscopic procedures. Surg Endosc 22:901–906
Supe A, Dalvi A, Prabhu R, Kantharia C, Bhuiyan P (2005) Cadaver as a model for laparoscopic training. Indian J Gastroenterol 24:111–113
Moglia A, Ferrari V, Morelli L, Ferrari M, Mosca F, Cuschieri A (2016) A systematic review of virtual reality simulators for robot-assisted surgery. Eur Urol 69:1065–1080
Ahmed K, Jawad M, Abboudi M et al (2011) Effectiveness of procedural simulation in urology: a systematic review. J Urol 186:26–34
Author information
Authors and Affiliations
Contributions
Protocol/project development: RM, SM, MB, AG. Data collection or management: BE, EB, PS, SF, RM, AG. Data analysis: BE, EB, PS, SF, RM, AG. Manuscript writing/editing: RM, AG, TC.
Corresponding author
Ethics declarations
Conflict of interest
RMelnyk: none. B Ezzat: none. E Belfast: none. P Saba: none. S Farooq: none. S McAleavey: none. M Buckley: none. A Ghazi: Intuitive Surgical: Research grant, Olympus America: Consultant.
Research involving human participants and/or animals
This research was conducted utilizing porcine kidneys. Fresh porcine kidneys were acquired through the University of Rochester veterinary research facilities.
Informed consent
No informed consent was required for this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised: The Eqs. (1), (2), (3) have been incorrectly moved, and separated into different sections. Now, it has been corrected.
Rights and permissions
About this article
Cite this article
Melnyk, R., Ezzat, B., Belfast, E. et al. Mechanical and functional validation of a perfused, robot-assisted partial nephrectomy simulation platform using a combination of 3D printing and hydrogel casting. World J Urol 38, 1631–1641 (2020). https://doi.org/10.1007/s00345-019-02989-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-019-02989-z