Abstract
Purpose
For patients with prostate cancer, validated and reliable instruments are essential for measuring patient-reported outcomes. The aim of this study was to validate the German version of the widely established Expanded Prostate Cancer Index Composite with 26 items (EPIC-26).
Methods
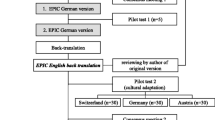
A German translation of the original questionnaire was tested in 3094 patients with localized or locally advanced (any T, any N and M0) prostate cancer with treatment intent (including radical prostatectomy, brachytherapy, active surveillance, watchful waiting). They completed the EPIC-26 questionnaire before treatment. A total of 521 of them also completed a questionnaire 12 months afterward. Internal consistency, sensitivity to change, and construct validity were assessed.
Results
The internal consistency of all domains was sufficient (Cronbach’s alpha between 0.64 and 0.93). Item-to-scale correlation coefficients showed acceptable associations between items and their domain score (all > 0.30), with the lowest scores for “bloody stools” (r = 0.37) and “breast problems” (r = 0.32). Confirmatory and exploratory factor analysis confirmed the five-dimension structure of the EPIC-26 (comparative fit index 0.95).
Conclusions
Psychometric evaluation suggests that the German version of the EPIC-26 is a well-constructed instrument for measuring patient-reported health-related symptoms in patients with prostate cancer.
Similar content being viewed by others
References
Zentrum für Krebsregisterdaten and Gesellschaft der epidemiologischen Krebsregister in Deutschland (2017) Krebs in Deutschland für 2013/2014. Robert Koch-Institut, Berlin
Trama A et al (2015) Survival of male genital cancers (prostate, testis and penis) in Europe 1999-2007: results from the EUROCARE-5 study. Eur J Cancer 51(15):2206–2216
Litwin MS et al (1995) Quality-of-life outcomes in men treated for localized prostate cancer. JAMA 273(2):129–135
Madalinska JB et al (2001) Health-related quality-of-life effects of radical prostatectomy and primary radiotherapy for screen-detected or clinically diagnosed localized prostate cancer. J Clin Oncol 19(6):1619–1628
Sanda MG et al (2008) Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med 358(12):1250–1261
Wei JT et al (2000) Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology 56(6):899–905
Szymanski KM et al (2010) Development and validation of an abbreviated version of the expanded prostate cancer index composite instrument for measuring health-related quality of life among prostate cancer survivors. Urology 76(5):1245–1250
Schmidt S et al (2014) Assessing quality of life in patients with prostate cancer: a systematic and standardized comparison of available instruments. Qual Life Res 23(8):2169–2181
Lam WWT et al (2017) Psychometric assessment of the Chinese version of the abbreviated expanded prostate cancer index composite (EPIC-26) and the clinical practice version (EPIC-CP) in chinese men with prostate cancer. J Pain Symptom Manage 53(6):1085–1090
Fossa SD et al (2016) Psychometric testing of the Norwegian version of the expanded prostate cancer index composite 26-item version (EPIC-26). Scand J Urol 50(4):280–285
Vigneault E et al (2017) Validation of the French-Canadian version of the expanded prostate cancer index composite (EPIC) in a French-Canadian population. Can Urol Assoc J 11(12):404–410
Beyer B et al (2015) “Expanded prostate cancer index composite” (EPIC-26): Funktionelles Behandlungsergebnis bei Patienten mit lokalisiertem Prostatakarzinom. Urologe A 54(11):1591–1595
Umbehr MH et al (2018) The German version of the Expanded Prostate Cancer Index Composite (EPIC): translation, validation and minimal important difference estimation. Health Qual Life Outcomes 16(1):36
Evans SM et al (2017) Cohort profile: the TrueNTH Global Registry: an international registry to monitor and improve localised prostate cancer health outcomes. BMJ Open 7(11):e017006
Martin NE et al (2015) Defining a standard set of patient-centered outcomes for men with localized prostate cancer. Eur Urol 67(3):460–467
ICHOM ( 2017) Localized prostate cancer data collection reference guide. https://ichom.org/files/medical-conditions/localized-prostate-cancer/localized-prostate-cancer-reference-guide.pdf. Accessed 23 Sep 2019
Cronbach LJ (1951) Coefficient alpha and the internal structure of tests. Psychometrika 16:297–334
Cohen J (1988) Statistical power analysis for the behavioral sciences. N.J.L. Erlbaum Associates, Hillsdale
Nunnally CJ, Bernstein I (1994) Psychometric Theory. McGraw-Hill, New York
Reeve Bryce B et al (2018) Symptom and function profiles of men with localized prostate cancer. Cancer 124(13):2832–2840
Nunnally JC, Bernstein IH (1994) Psychometric theory, 3rd edn. McGraw-Hill, New York
German Guideline Program in Oncology (2018) ed. Interdisziplinäre Leitlinie der Qualität S3 zur Früherkennung, Diagnose und Therapie der verschiedenen Stadien des Prostatakarzinoms, Version 5.0, 1. Aktualisierung, AWMF-Registernummer: 043/022OL
Wei J, Dunn R, Litwin M, Sandler H, and Sanda M (2000) Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology 56:899–905
Funding
This study was funded by the Movember Foundation.
Author information
Authors and Affiliations
Contributions
NT Sibert: data analysis, data collection and management, manuscript writing/editing; S Dieng: protocol/project development, data collection and management, manuscript writing/editing; A Oesterle: data analysis, data collection and management; G Feick: protocol/project development, manuscript writing/editing; G Carl: protocol/project development, manuscript writing/editing; T Steiner: data collection and management, manuscript writing/editing; J Minner: data collection and management, manuscript writing/editing; F Roghmann: data collection and management, manuscript writing/editing; B Kaftan: data collection and management, manuscript writing/editing; F Zengerling: B Kaftan: data collection and management, manuscript writing/editing; A Hinkel: data collection and management, manuscript writing/editing; B Beyer: data collection and management, manuscript writing/editing; A Heidenreich: data collection and management, manuscript writing/editing; N Harke: data collection and management, manuscript writing/editing; B Brehmer: data collection and management, manuscript writing/editing; J Pfitzenmaier: data collection and management, manuscript writing/editing; J Fichtner: data collection and management, manuscript writing/editing; A Neisius: data collection and management, manuscript writing/editing; P Hammerer: data collection and management, manuscript writing/editing; S Wesselmann: protocol/project development, manuscript writing/editing; C Kowalski: protocol/project development, data analysis, manuscript writing/editing.
Corresponding author
Ethics declarations
Conflicts of interest
GC, GF, TS, JM, FR, FZ, AHi, BB, AHe, NH, BB, JP, JF, AN, and PH hereby declare that they have no potential conflicts of interest. NTS, CK, SD, AO, and SW are employees of the two institutions in charge of the certification system. BK receives consulting and lecturing honoraria from various companies that do not involve any conflicts with the research presented here. A comprehensive list is available on request.
Research involving human participants and/or animals
All procedures performed in studies involving human participants were in accordance with the ethical standards of the ethics committee of the Medical Association of Berlin (Eth-12/16) and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix 1: Comparison of the four-, five- and six-factor solutions
Appendix 2: The EPIC-26—Expanded Prostate Cancer Index Composite Short Form [23]



Appendix 3: German translation of the EPIC-26 [12]



Appendix 4: Staging according to the German Guideline Prostate Cancer (version April 2018)
In this study we use the staging proposed by the German Guideline for Prostate Cancer [22]:
-
Localized prostate cancer: T1-2, N0 M0.
-
Localized prostate cancer with low risk: PSA ≤ 10 ng/ml and Gleason-Score 6 and cT1c or cT2a.
-
Localized prostate cancer with intermediate risk: PSA > 10 ng/ml–30 ng/ml or Gleason 7 or cT 2b.
-
Localized prostate cancer with high risk: PSA > 20 ng/ml or Gleason ≥ 8 or cT2c.
-
-
Locally advanced prostate cancer: T3-4 N0 M0.
-
Advanced prostate cancer: any T N1 and M0.
-
Metastasized prostate cancer: any T any N and M1.
Furthermore, localized prostate cancer with cT1a or cT1b is classified as localized prostate cancer with low risk.
Rights and permissions
About this article
Cite this article
Sibert, N.T., Dieng, S., Oesterle, A. et al. Psychometric validation of the German version of the EPIC-26 questionnaire for patients with localized and locally advanced prostate cancer. World J Urol 39, 11–25 (2021). https://doi.org/10.1007/s00345-019-02949-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-019-02949-7