Abstract
Purpose
To combine multiparametric MRI (mpMRI) findings and clinical parameters to provide nomograms for diagnosing different scenarios of aggressiveness of prostate cancer (PCa).
Methods
A cohort of 346 patients with suspicion of PCa because of abnormal finding in digital rectal examination (DRE) and/or high prostate specific antigen (PSA) level received mpMRI prior to prostate biopsy (PBx). A conventional 12-core transrectal PBx with two extra cores from suspicious areas in mpMRI was performed by cognitive fusion. Multivariate logistic regression analysis was performed combining age, PSA density (PSAD), DRE, number of previous PBx, and mpMRI findings to predict three different scenarios: PCa, significant PCa (ISUP-group ≥ 2), or aggressive PCa (ISUP-group ≥ 3). We validate models by ROC curves, calibration plots, probability density functions (PDF), and clinical utility curves (CUC). Cut-off probabilities were estimated for helping decision-making in clinical practice.
Results
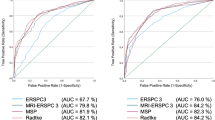
Our cohort showed 39.6% incidence of PCa, 32.6% of significant PCa, and 23.4% of aggressive PCa. The AUC of predictive models were 0.856, 0.883, and 0.911, respectively. The PDF and CUC showed 11% missed diagnoses of significant PCa (35 cases of 326 significant PCa expected in 1000 proposed Bx) when choosing < 18% as the cutoff of probability for not performing PBx; the percentage of saved PBx was 47% (474 avoided PBx in 1000 proposed).
Conclusion
We developed clinical and mpMRI-based nomograms with a high discrimination ability for three different scenarios of PCa aggressiveness (https://urostatisticalsolutions.shinyapps.io/MRIfusionPCPrediction/). Specific clinical cutoff points allow us to save a high number of PBx with a minimum of missed diagnoses.





Similar content being viewed by others
References
Siegel R, Miller KD, Ahmedin J (2017) Cáncer statistics. Ca Cáncer J 67:7–30. https://doi.org/10.3322/caac.21387
Galceran J, Ameijide A, Carulla M, Mateos A, Quirós JR et al (2017) Cancer incidence in Spain, 2015. Clin Transl Oncol 19:799–825. https://doi.org/10.1007/s12094-016-1607-9
Li M, Huang Z, Yu H, Wang Y, Zhang Y, Song B (2019) Comparison of PET/MRI with multiparametric MRI in diagnosis of primary prostate cancer: a meta-analysis. Eur J Radiol 113:225–231. https://doi.org/10.1016/j.ejrad.2019.02.028
Cuocolo R, Stanzione A, Rusconi G, Petretta M, Ponsiglione A, Fusco F, Longo N, Persico F, Cocozza S, Brunetti A, Imbriaco M (2018) PSA-density does not improve bi-parametric prostate MR detection of prostate cancer in a biopsy naïve patient population. Eur J Radiol 104:64–70. https://doi.org/10.1016/j.ejrad.2018.05.004
Sanda MG, Cadeddu J, Kirkby E, Chen RC, Crispino T, Fontanarosa J, Freedland SJ, Greene K, Klotz LH, Makarov DV, Nelson JB, Rodrigues G, Sandler HM, Taplin ME, Treadwell JR (2018) Clinically localized prostate cancer: AUA/ASTRO/SUO guideline. Part II: recommended approaches and details of specific care options. J Urol 199:990–997. https://doi.org/10.1016/j.juro.2018.01.002
Carroll PR, Parsons JK, Andriole G, Bahnson RR, Carlsson S, Castle EP, Catalona WJ, Dahl DM, Davis JW, Epstein JI, Etzioni RB, Farrington T, Hemstreet GP, Jarrard D, Kibel AS, Kim S, Lowrance W, Maroni P, Mohler J, Morgan TM, Moses KA, Nadler RB, Poch M, Scales C, Shaneyfelt TM, Smaldone MC, Sonn G, Sprenkle P, Wake R, Yuh B (2019) NCCN clinical practice guidelines in oncology (NCCN Guidelines®) Prostate Cancer Early Detection Version 1.2019. https://www.nccn.org/professionals/physician_gls/pdf/prostate_detection.pdf. Accessed 14 May 2019
Mohler JL, Antonarakis ES, Armstrong AJ, D’Amico AV, Davis BJ, Dorff T, Eastham JA, Enke CA, Farrington T, Ellis R, Higano CS, Horwitz EM, Hurwitz M, Ippolito JE, Kane CJ, Kuettel ME, Lang JM, Netto G, Penson DF, Plimack ER, Pow-Sang JM, Pugh TJ, Richey S, Roach III M, Rosenfeld S, Schaeffer E, Shabsigh A, Small EJ, Spratt DE, Srinivas S, Tward J (2019) NCCN clinical practice guidelines in oncology (NCCN Guidelines®) Prostate Cancer Version 1.2019. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Accessed 14 May 2019
Mottet N, van den Bergh R, Briers E, Cornford P, De Santis M, Fanti S, Gillesen S, Grummel J, Henry AM, Lam TB, Mason MD, van der Kwast T, van der Poel HG, Rouvière O, Tilki D, Wiegel T, van der B T, Cumberbatch M, Fossati N, Gross T, Lardas M, Liew M, Moris L, Schoots IG, Willemse PM (2019) EAU-EANM-ESTRO-ESUR-SIOG, guidelines on prostate cancer. 2019. https://uroweb.org/guideline/prostate-cancer/#3. Accessed 14 May 2019
PI-RADS TM prostate imaging—reporting and data system (2015). https://www.acr.org/-/media/ACR/Files/RADS/Pi-RADS/PIRADS-V2.pdf?la=en. Accessed 14 May 2019
Weinreb JC, Barentsz JO, Choyke PL, Cornud F, Haider MA, Macura KJ, Margolis D, Schnall MD, Shtern F, Tempany CM, Thoeny HC, Verma S, Catto J (2016) PI-RADS prostate imaging—reporting and data system: 2015, Version 2. Eur Urol 69:16–40. https://doi.org/10.1016/j.eururo.2015.08.052
Schaudinn A, Gawlitza J, Mucha S, Linder N, Franz T, Horn LC, Kahn T, Busse H (2019) Comparison of PI-RADS v1 and v2 for multiparametric MRI detection of prostate cancer with whole-mount histological workup as reference standard. Eur J Radiol. https://doi.org/10.1016/j.ejrad.2019.04.012
Epstein JI, Zelefsky MJ, Sjoberg DD, Nelson JB, Egevad L, Magi-Galluzzi C, Vickers AJ, Parwani AV, Reuter VE, Fine SW, Eastham JA, Wiklund P, Han M, Reddy CA, Ciezki JP, Nyberg T, Klein EA (2016) A contemporary prostate cancer grading system: a validated alternative to the Gleason score. Eur Urol 69:428–435. https://doi.org/10.1016/j.eururo.2015.06.046
Humphrey PA, Moch H, Cubilla AL, Ulbright TM, Reuter VE (2016) The 2016 WHO classification of tumours of the urinary system and male genital organs part B: prostate and bladder tumours. Eur Urol 70:106–119. https://doi.org/10.1016/j.eururo.2016.02.028
Rubio-Briones J, Borque A, Esteban LM, Casanova J, Fernandez-Serra A, Rubio L, Casanova-Salas I, Sanz G, Domínguez-Escrig J, Collado A, Gómez-Ferrer A, Iborra I, Ramírez-Backhaus M, Martínez F, Calatrava A, Lopez-Guerrero JA (2015) Optimizing the clinical utility of PCA3 to diagnose prostate cancer in initial prostate biopsy. BMC Cancer 15:633. https://doi.org/10.1186/s12885-015-1623-0
Borque-Fernando A, Esteban-Escaño LM, Rubio-Briones J, García-Ruiz R, Tejero-Sánchez A, Muñoz-Rivero MT, Alfaro-Torres J, Marquina-Ibáñez IM, Hakim-Alonso S, Mejía-Urbáez E, Gil-Fabra J, Gil-Martínez P, Álvarez-Alegret R, Sanz G, Gil-Sanz MJ (2017) A preliminary study of the ability of the 4Kscore test, the Prostate Cancer Prevention Trial-Risk Calculator and the European Research Screening Prostate-Risk Calculator for predicting high-grade prostate cancer. Actas Urol Esp 40:155–163
Borque A, Rubio-Briones J, Esteban LM, Sanz G, Domínguez-Escrig J, Ramírez-Backhaus M, Calatrava A, Solsona E (2014) Implementing the use of nomograms by choosing threshold points in predictive models: 2012 updated Partin Tables vs a European predictive nomogram for organ-confined disease in prostate cancer. BJU Int 113:878–886. https://doi.org/10.1111/bju.12532
Bjurlin MA, Rosenkrantz AB, Sarkar S, Lepor H, Huang WC, Huang R, Venkataraman R, Taneja SS (2018) Prediction of prostate cancer risk among men undergoing combined MRI-targeted and systematic biopsy using novel pre-biopsy nomograms that incorporate MRI findings. Urology 112:112–120. https://doi.org/10.1016/j.jchromb.2016.09.003
van Leeuwen PJ, Hayen A, Thompson JE, Moses D, Shnier R, Böhm M, Abuodha M, Haynes AM, Ting F, Barentsz J, Roobol M, Vass J, Rasiah K, Delprado W, Stricker PD (2017) A multiparametric magnetic resonance imaging-based risk model to determine the risk of significant prostate cancer prior to biopsy. BJU Int 120:774–781. https://doi.org/10.1111/bju.13814
Radtke JP, Bonekamp D, Kesch C, Freitag MT, Alt CD, Celik K, Distler F, Roth W, Wieczorek K, Duensing S, Roethke MC, Teber D, Schlemmer HP, Hohenfellner M, Hadaschik BA, Wiesenfarth M, Stock C (2017) Combined clinical parameters and multiparametric MRI for advanced risk modeling of prostate cancer—patient-tailored risk stratification can reduce unnecessary biopsies. Eur Urol 72:888–896. https://doi.org/10.1016/S1569-9056(17)30557-2
Mehralivand S, Shih JH, Rais-Bahrami S, Oto A, Bednarova S, Nix JW, Thomas JV, Gordetsky JB, Gaur S, Harmon SA, Siddiqui MM, Merino MJ, Parnes HL, Wood BJ, Pinto PA, Choyke PL, Turkbey B (2018) A magnetic resonance imaging-based prediction model for prostate biopsy risk stratification. JAMA Oncol 4:678–685. https://doi.org/10.1001/jamaoncol.2017.5667
Alberts AR, Roobol MJ, Verbeek FJM, Schoots IG, Chiu PK, Osses F, Tijsterman JD, Beerlage HP, Mannaerts CK, Albers P, Arsov C, Schimmo L, Novara G, Vickers A (2019) Prediction of high-grade prostate cancer following multiparametric magnetic resonance imaging: improving the rotterdam european randomized study of screening for prostate cancer risk calculators. Eur Urol 75:310–318. https://doi.org/10.1016/j.eururo.2018.07.031
Rosenkrantz AB, Kim S, Lim RP, Hindman N, Deng FM, Babb JS, Taneja SS (2013) Prostate cancer localization using multiparametric MR imaging: comparison of Prostate Imaging Reporting and Data System (PI-RADS) and Likert scales. Radiology 269:482–492. https://doi.org/10.1148/radiol.13122233/-/DC1
Wysock JS, Rosenkrantz AB, Huang WC, Stifelman MD, Lepor H, Deng FM, Melamed J, Taneja SS (2014) A prospective, blinded comparison of magnetic resonance (MR) imaging-ultrasound fusion and visual estimation in the performance of MR-targeted prostate biopsy: the profus trial. Eur Urol 66:343–351. https://doi.org/10.1016/j.eururo.2013.10.048
Barentsz JO, Richenberg J, Clements R, Choyke P, Verma S, Villeirs G, Rouviere O, Logager V, Fütterer JJ (2012) ESUR prostate MR guidelines 2012. Eur Radiol. https://doi.org/10.1007/s00330-011-2377-y
Borque-Fernando A, Esteban LM, Morote-Robles J, Sanz G (2019) WHO Predictive model. https://urostatisticalsolutions.shinyapps.io/FusionBiopsy/. Accessed 14 May 2019
Chun FK, de la Taille A, van Poppel H, Marberger M, Stenzl A, Mulders PFA, Huland H, Abbou CC, Stillebroer AB, van Gils MP, Schalken JA, Fradet Y, Marks LS, Ellis W, Partin AW, Haese A (2009) Prostate Cancer Gene 3 (PCA3): development and internal validation of a novel biopsy nomogram. Eur Urol 56:659–668. https://doi.org/10.1016/j.eururo.2009.03.029
Lughezzani G, Lazzeri M, Larcher A, Lista G, Scattoni V, Cestari A, Buffi NM, Bini V, Guazzoni G (2012) Development and internal validation of a prostate health index based nomogram for predicting prostate cancer at extended biopsy. J Urol 188:1144–1150. https://doi.org/10.1016/j.juro.2012.06.025
Parekh DJ, Punnen S, Sjoberg DD, Asroff SW, Bailen JL, Cochran JS, Concepcion R, David RD, Deck KB, Dumbadze I, Gambla M, Grable MS, Henderson RJ, Karsh L, Krisch EB, Langford TD, Lin DW, McGee SM, Munoz JJ, Pieczonka CM, Rieger-Christ K, Saltzstein DR, Scott JW, Shore ND, Sieber PR, Waldmann TM, Wolk FN, Zappala SM (2015) A multi-institutional prospective trial in the USA confirms that the 4Kscore accurately identifies men with high-grade prostate cancer. Eur Urol 68:464–470. https://doi.org/10.1016/j.eururo.2014.10.021
Thompson IM, Ankerst DP, Chi C, Goodman PJ, Tangen CM, Lucia MS, Feng Z, Parnes HL, Coltman CA (2006) Assessing prostate cancer risk: results from the Prostate Cancer Prevention Trial. J Natl Cancer Inst 98:529–534. https://doi.org/10.1093/jnci/djj131
Roobol MJ, Van Vugt HA, Loeb S, Zhu X, Bul M, Bangma CH, Van Leenders AG, Steyerberg EW, Schröder FH (2012) Prediction of prostate cancer risk: The role of prostate volume and digital rectal examination in the ERSPC risk calculators. Eur Urol 61:577–583. https://doi.org/10.1016/j.eururo.2011.11.012
Funding
No funding was received for this work.
Author information
Authors and Affiliations
Contributions
AB-F: project development, data analysis, manuscript writing. LME: project development, data analysis, manuscript writing. AC: project development, data collection, manuscript writing. SR: project development, data collection, manuscript writing. JP: project development, data collection, manuscript writing. LR: project development, data collection, manuscript writing. IT: project development, data collection, manuscript writing. MES: project development, data collection, manuscript writing. ET: project development, data collection, manuscript writing. JM: project development, data collection, manuscript writing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest from any of the co-authors.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Patients prospectively collected signed informed consent form.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Borque-Fernando, Á., Esteban, L.M., Celma, A. et al. How to implement magnetic resonance imaging before prostate biopsy in clinical practice: nomograms for saving biopsies. World J Urol 38, 1481–1491 (2020). https://doi.org/10.1007/s00345-019-02946-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-019-02946-w