Abstract
Purpose
This trial assessed if written information on procedural findings and subsequent treatment improved understanding and reduced anxiety among patients undergoing day case flexible cystoscopy (FC).
Methods
Participants completed pre- and post-procedure questionnaires self-rating anxiety and feeling well informed on 5-point Likert scales. Supplemental written information was provided after FC to half the patients on a standardized template, according to randomized allocation. Comparisons between the groups were undertaken using the Wilcoxon test.
Results
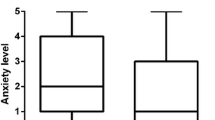
Two hundred patients were recruited, with 171 evaluable questionnaires (83 from written group). The distribution of age, sex and prior FC, as well as the pre-procedure self-assessment of anxiety and understanding, was similar between the two groups. Patients receiving written information reported feeling better informed, with median (range) Likert score of 5 (4–5) compared to 4 (1–5) out of 5 (p < 0.0001) and less anxious (score 1 [1–4] compared to 2 [1–5] out of 5, p < 0.005), although all except four patients had an accurate understanding of the information provided (p = NS).
Conclusions
Written information at the time of FC leads to patients feeling better informed and less anxious, although verbal information alone appears to lead to an adequate understanding.
Clinical trial registration number
ACTRN12616000288426

Similar content being viewed by others
References
Greenwood J (2002) Employing a range of methods to meet patient information needs. Prof Nurse 18:233–236
Macfarlane J, Holmes W, Gard P, Thornhill D, Macfarlane R, Hubbard R (2002) Reducing antibiotic use for acute bronchitis in primary care: blinded, randomised controlled trial of patient information leaflet. BMJ 324:91–94
Wood D, Deshpande A, Wijewardena M, Gujral S (2006) A study of how urology out-patients like to receive clinical information. Ann R Coll Surg Engl 88:579–582
The Department of Health (2000) The NHS Plan: a Plan For Investment, a Plan For Reform. HMSO, Norwich
Angioli R, Plotti F, Capriglione S et al (2014) The effects of giving patients verbal or written pre-operative information in gynecologic oncology surgery: a randomized study and the medical-legal point of view. Eur J Obstet Gynecol Reprod Biol 177:67–71
Sheard C, Garrud P (2006) Evaluation of generic patient information: effects on health outcomes, knowledge and satisfaction. Patient Educ Couns 61:43–47
Kirby R, Challacombe B, Hughes S, Chowdhury S, Dasgupta P (2013) Increasing importance of truly informed consent: the role of written patient information. BJU Int 112:715–716
Masood J, Hafeez A, Wiseman O, Hill JT (2007) Informed consent: are we deluding ourselves? A randomized controlled study. BJU Int 99:4–5
Blandford CM, Gupta BC, Montgomery J, Stocker ME (2011) Ability of patients to retain and recall new information in the post-anaesthetic recovery period: a prospective clinical study in day surgery. Anaesthesia 66:1088–1092
Murphy S, Conway C, McGrath NB, O’Leary B, O’Sullivan MP, O’Sullivan D (2011) An intervention study exploring the effects of providing older adult hip fracture patients with an information booklet in the early postoperative period. J Clin Nurs 20:3404–3413
Davison BJ, Keyes M, Elliott S, Berkowitz J, Goldenberg SL (2004) Preferences for sexual information resources in patients treated for early-stage prostate cancer with either radical prostatectomy or brachytherapy. BJU Int 93:965–969
Binhas M, Roudot-Thoraval F, Thominet D, Maison P, Marty J (2008) Impact of written information describing postoperative pain management on patient agreement with proposed treatment. Eur J Anaesthesiol 25:884–890
Bellani ML (2008) Psychological aspects in day-case surgery. Int J Surg 6:S44–S46
Mulsow JJ, Feeley TM, Tierney S (2012) Beyond consent - improving understanding in surgical patients. Am J Surg 203:112–120
Dy GW, Gore JL, Muncey WW, Ellison JS, Merguerian PA. (2017) Comparative effectiveness of a pilot patient-centered ultrasound report in the management of hydronephrosis. J Pediatr Urol. https://doi.org/10.1016/j.jpurol.2017.08.014. [Epub ahead of print]
Mossanen M, Macleod LC, Chu A, Wright JL, Dalkin B, Lin DW, True L, Gore JL (2016) Comparative effectiveness of a patient centered pathology report for bladder cancer care. J Urol 196(5):1383–1389
Bollschweiler E, Apitzsch J, Obliers R et al (2008) Improving informed consent of surgical patients using a multimedia-based program? Results of a prospective randomized multicenter study of patients before cholecystectomy. Ann Surg 248:205–211
Gyomber D, Lawrentschuk N, Wong P, Parker F, Bolton DM (2010) Improving informed consent for patients undergoing radical prostatectomy using multimedia techniques: a prospective randomized crossover study. BJU Int 106:1152–1156
Astley CM, Chew DP, Aylward PE, Molloy DA, De Pasquale CG (2008) A randomised study of three different informational AIDS prior to coronary angiography, measuring patient recall, satisfaction and anxiety. Heart Lung Circ 17:25–32
Author information
Authors and Affiliations
Contributions
FD’A: study procedures and implementation, and manuscript preparation. CLY and KM: protocol development and data collection. PM and RM: study procedures and implementation. DB: protocol development and infrastructure support. SS: protocol development, data collection and analysis, and manuscript preparation.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they do not have any conflicts of interest to report.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
D’Arcy, F., Yip, C.L., Manya, K. et al. Prospective randomised controlled trial of written supplement to verbal communication of results to patients at the time of flexible cystoscopy. World J Urol 36, 883–887 (2018). https://doi.org/10.1007/s00345-018-2233-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-018-2233-7