Abstract
Purpose
This study aimed at reporting the long-term oncological outcomes of robotic partial nephrectomy (RPN) for renal cell carcinoma (RCC).
Methods
Data from all consecutive patients who underwent RAPN for RCC from July 2009 to January 2012 in three departments of urology were prospectively collected. Overall survival (OS), cancer-specific survival (CSS) and disease free-survival (DFS) were estimated using the Kaplan–Meier method. Prognostic factors associated with CSS were sought in univariate analysis. The log-rank test was used for categorical variables and the Cox model for continuous variables.
Results
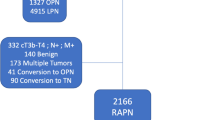
110 patients were included with a median follow-up of 64.4 months [95% CI = (61.0–66.7)]. Median age was 61 years (29–83) with 62.7% of men and 37.3% of women. Median RENAL score was 6 (4–10) with elective indications accounting for 95% of cases. Out of 27 patients (24.5%) who experienced peri-operative complication, 12 patients (10.9%) had a major complication (Clavien-Dindo grade ≥ 3). The TRIFECTA achievement rate was 52.7%. Three patients (2.7%) experienced local recurrence and seven patients (6.4%) progressed to a metastatic disease. 5-year OS, CSS, DFS were 94.9, 96.8, 86.4%, respectively. In univariate analysis, no pre/peri-operative characteristic was associated with DFS. No port-site metastasis was observed and there was one case of peritoneal carcinomatosis.
Conclusion
In this multicenter series, long-term OS, DFS and CSS after RPN appeared comparable to large series of open partial nephrectomy, with no port-site metastasis and one case of peritoneal carcinomatosis.

Similar content being viewed by others
References
Smittenaar CR, Petersen KA, Stewart K, Moitt N (2016) Cancer incidence and mortality projections in the UK until 2035. Br J Cancer 115:1147–1155
Ljungberg B, Bensalah K, Canfield S et al (2015) EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol 67:913–924
Zini L, Perrotte P, Capitanio U et al (2009) Radical versus partial nephrectomy: effect on overall and noncancer mortality. Cancer 115:1465–1471
Gill IS, Kavoussi LR, Lane BR et al (2007) Comparison of 1800 laparoscopic and open partial nephrectomies for single renal tumors. J Urol 178:41–46
Marszalek M, Meixl H, Polajnar M, Rauchenwald M, Jeschke K, Madersbacher S (2009) Laparoscopic and open partial nephrectomy: a matched-pair comparison of 200 patients. Eur Urol 55:1171–1178
Lane BR, Gill IS (2010) 7-year oncological outcomes after laparoscopic and open partial nephrectomy. J Urol 183:473–479
Choi JE, You JH, Kim DK, Rha KH, Lee SH (2015) Comparison of perioperative outcomes between robotic and laparoscopic partial nephrectomy: a systematic review and meta-analysis. Eur Urol 67:891–901
Masson-Lecomte A, Yates DR, Hupertan V et al (2013) A prospective comparison of the pathologic and surgical outcomes obtained after elective treatment of renal cell carcinoma by open or robot-assisted partial nephrectomy. Urol Oncol 31:924–929
Khalifeh A, Autorino R, Eyraud R et al (2013) Three-year oncologic and renal functional outcomes after robot-assisted partial nephrectomy. Eur Urol 64:744–750
Peyronnet B, Seisen T, Oger E et al (2016) Comparison of 1800 robotic and open partial nephrectomies for renal tumors. Ann Surg Oncol 23:4277–4283
Andrade HS, Zargar H, Caputo PA et al (2016) Five-year oncologic outcomes after transperitoneal robotic partial nephrectomy for renal cell carcinoma. Eur Urol 69:1149–1154
Kutikov A, Uzzo RG (2009) The R.E.N.A.L. nephrometry score: a comprehensive standardized system for quantitating renal tumor size, location and depth. J Urol 182:844–853
Wolters U, Wolf T, Stutzer H, Schroder T (1996) ASA classification and perioperative variables as predictors of postoperative outcome. Br J Anaesth 77:217–222
Zargar H, Allaf ME, Bhayani S et al (2015) Trifecta and optimal perioperative outcomes of robotic and laparoscopic partial nephrectomy in surgical treatment of small renal masses: a multi-institutional study. BJU Int 116:407–414
Lopez-Beltran A, Scarpelli M, Montironi R, Kirkali Z (2006) 2004 WHO classification of the renal tumors of the adults. Eur Urol 49:798–805
Patard JJ, Baumert H, Bensalah K et al (2013) CCAFU recommendations 2013: renal cancer. Progres en urologie: Journal de l’Association francaise d’urologie et de la Societe francaise d’urologie 23(Suppl 2):S177–S204
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213
Kyllo RL, Tanagho YS, Kaouk JH et al (2012) Prospective multi-center study of oncologic outcomes of robot-assisted partial nephrectomy for pT1 renal cell carcinoma. BMC Urol 12:11
Marszalek M, Carini M, Chlosta P et al (2012) Positive surgical margins after nephron-sparing surgery. Eur Urol 61:757–763
Bernhard JC, Pantuck AJ, Wallerand H et al (2010) Predictive factors for ipsilateral recurrence after nephron-sparing surgery in renal cell carcinoma. Eur Urol 57:1080–1086
Khalifeh A, Kaouk JH, Bhayani S et al (2013) Positive surgical margins in robot-assisted partial nephrectomy: a multi-institutional analysis of oncologic outcomes (leave no tumor behind). J Urol 190:1674–1679
Borghesi M, Brunocilla E, Schiavina R, Martorana G (2013) Positive surgical margins after nephron-sparing surgery for renal cell carcinoma: incidence, clinical impact, and management. Clin Genitourin Cancer 11:5–9
Bensalah K, Pantuck AJ, Rioux-Leclercq N et al (2010) Positive surgical margin appears to have negligible impact on survival of renal cell carcinomas treated by nephron-sparing surgery. Eur Urol 57:466–471
Ristau BT, Kutikov A, Uzzo RG, Smaldone MC (2016) Active surveillance for small renal masses: when less is more. Eur Urol Focus 2(6):660–668
Prins FM, Kerkmeijer LGW, Pronk AA et al (2017) Renal cell carcinoma: alternative nephron-sparing treatment options for small renal masses, a systematic review. J Endourol 31(10):963–975
Author information
Authors and Affiliations
Contributions
JBB: Protocol/project development, Data analysis, Manuscript writing/editing. BP: Protocol/project development, Data analysis, Manuscript writing/editing. TB: Data collection or management, Data analysis. BC: Data analysis. TS: Manuscript writing/editing. MR: Protocol/Project development. BP: Data collection or management, Data analysis. ZEK: Data collection or management, Manuscript writing/editing. QM: Data collection or management. GV: Manuscript writing/editing. MT: Manuscript writing/editing. JP: Data collection or management. ND: Manuscript writing/editing. RM: Protocol/Project development. CV: Data collection or management, Data analysis. MS: Protocol/project development, Data analysis, Manuscript writing/editing. MR: Data collection or management, Data analysis, Manuscript writing/editing. KB: Manuscript writing/editing, Protocol/project development, Data collection or management, Data analysis.
Corresponding author
Ethics declarations
Conflict of interest
Karim Bensalah, Christophe Vaessen, Nicolas Doumerc and Gregory Verhoest are proctors for Intuitive Surgical®. Other authors declare that they have no conflict of interest.
Ethical standards
All participants have given informed consent before inclusion in the present study.
Rights and permissions
About this article
Cite this article
Beauval, JB., Peyronnet, B., Benoit, T. et al. Long-term oncological outcomes after robotic partial nephrectomy for renal cell carcinoma: a prospective multicentre study. World J Urol 36, 897–904 (2018). https://doi.org/10.1007/s00345-018-2208-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-018-2208-8