Abstract
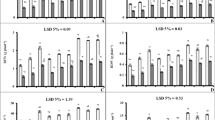
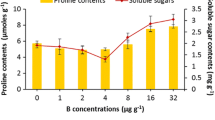
A study was conducted to assess the influence of boron (B) toxicity on functioning of antioxidant machinery to counteract oxidative stress in maize (Zea mays L.) plants as well as the mitigating effect of kinetin (KIN) and indole acetic acid (IAA) on these phenomena. Plants of maize cv. DK 647 F1 were exposed to 0.05 and 2 mM boron in nutrient solution 8 days after germination, and the plants were grown for a further 7 days in these conditions. After 15 days growth, deionized water (control), 1.0 or 2.0 mM of KIN, or IAA were applied to the leaves of maize plants once each 7 days. After 21 days of these treatments, the plants were harvested to evaluate growth, water relations, and oxidative and antioxidative systems. Boron toxicity significantly reduced dry matter, efficiency of photosystem II (Fv/Fm), and leaf relative water content in the maize plants when compared to those in non-stressed plants, but in contrast, it enhanced electrolyte leakage (EL), hydrogen peroxide (H2O2), free proline, malondialdehyde (MDA) and the activities of peroxidase, superoxide dismutase, and catalase in the maize plants. However, KIN or IAA applied as a foliar spray to maize plants grown at excess B caused a significant improvement in growth attributes, plant water status and the activities of various antioxidant enzymes as well as proline content, but they lowered EL, and H2O2 and MDA contents. Boron toxicity increased leaf B and reduced leaf K+, Ca2+, and P contents when compared to those in the control plants. Foliar applied KIN or IAA to the plant leaves lowered tissue B levels, but in contrast, it resulted in significant increases in Ca2+, K+ and P levels. The results of the study indicated that the spray of KIN and IAA, particularly at 2 mM, can mitigate to a significant extent the adverse effects of B toxicity on maize plants, which was found be associated with reduced content of B, H2O2, MDA as well as EL, and increased activities of key antioxidant enzymes in maize plants.
Similar content being viewed by others
References
Afzal A, Basra SMA, Iqbal A (2005) The effects of seed soaking with plant growth regulators on seedling vigor of wheat under salinity stress. J Plant Physiol Biochem 1(1):6–14
Ahammed GJ, Choudhary SP, Chen S, Xia X, Shi K, Zhou Y, Yu J (2013) Role of brassinosteroids in alleviation of phenanthrene-cadmium co-contamination-induced photosynthetic inhibition and oxidative stress in tomato. J Exp Bot 64:199–213
Ahmad I, Mohammad F, Taj S, Munsif F, Akber S (2017) Performance of F1 hybrids in sunflower (Helianthus annuus L.) under the environmental conditions of Peshawar valley. Int J Agric Environ Res 3(1):112–115
Akram NA, Ashraf M (2011) Pattern of accumulation of inorganic elements in sunflower (Helianthus annuus L.) plants subjected to salt stress and exogenous application of 5-aminolevulinic acid. Pak J Bot 43(1):521–530
Akram NA, Iqbal M, Ashraf M, Al-Qurainy F, Shafiq S (2018) Aminolevulinic acid and nitric oxide regulate oxidative defense and secondary metabolisms in canola (Brassica napus L.) under drought stress. Protoplasma 255(1):163–174
Aldesuquy H, Baka Z, Mickky B (2014) Kinetin and spermine mediated induction of salt tolerance in wheat plants: leaf area, photosynthesis and chloroplast ultra structure of flag leaf at ear emergence. Egypt J Basic Appl Sci 1:77–87
Al-Hakimi AMA (2007) Modification of cadmium toxicity in pea seedlings by kinetin. Plant Soil Environ 53:129–135
Ali RM, Abbas HM (2003) Response of salt stressed barley seedlings to phenylurea. Plant Soil Environ 49:158–162
Alpaslan M, Gunes A (2001) Interactive effects of boron and salinity stress on the growth, membrane permeability and mineral composition of tomato and cucumber plants. Plant Soil 236:123–128
Ambler JR, Morgan PW, Jordan WR (1992) Amounts of zeatin and zeatin riboside in xylem sap of senescent and non senescent sorghum. Crop Sci 32:411–419
Asgher M, Khan MIR, Anjum NA, Khan NA (2015) Minimizing toxicity of cadmium in plants–role of plant growth regulators. Protoplasma 252:399–413
Ashraf M (2009) Biotechnological approach of improving plant salt tolerance using antioxidants as markers. Biotechnol Adv 27(1):84–93
Ashraf M, Harris PJC (2013) Photosynthesis under stressful environments: an overview. Photosynthetica 51(2):163–190
Ashraf M, Akram NA, Arteca RN, Foolad MR (2010) The physiological, biochemical and molecular roles of brassinosteroids and salicylic acid in plant processes and salt tolerance. Crit Rev Plant Sci 29(3):162–190
Ashraf M, Akram NA, Al-Qurainy F, Foolad MR (2011) Drought tolerance: roles of organic osmolytes, growth regulators and mineral nutrients. Adv Agron 111:249–296
Ayvaz M, Guven A, Fagerstedt K (2015) Does excess boron affect hormone levels of potato cultivars? Biotechnol Equip 29(5):887–891
Aziz A, Akram NA, Ashraf M (2018) Influence of natural and synthetic vitamin C (ascorbic acid) on primary and secondary metabolites and associated metabolism in quinoa (Chenopodium quinoa Willd.) plants under water deficit regimes. Plant Physiol Biochem 123:192–203
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–207
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287
Bielach A, Hrtyan M, Tognetti VB (2017) Review plants under stress: involvement of auxin and cytokinin. Int J Mol Sci 18(7):1427
Bradford MM (1976) A rapid and sensitive method for the quantitation of micro gram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Chance B, Maehly C (1955) Assay of catalase and peroxidases. Methods Enzymol 2:764–775
Chapman HD, Pratt PF (1982) Methods of plant analysis. I. Methods of analysis for soils. Plants and Water Chapman Publishers, Riverside
Donati AJ, Lee HI, Leveau JH, Chang WS (2013) Effects of indole-3-acetic acid on the transcriptional activities and stress tolerance of Bradyrhizobium japonicum. PLoS ONE 8:e76559
Eggert K, Wirén N (2017) Response of the plant hormone network to boron deficiency. New Phytol 216(3):868–881
Eser A, Aydemir T (2016) The effect of kinetin on wheat seedlings exposed to boron. Plant Physiol Biochem 108:158–164
Fantozzi F, Bartocci P, D’Alessandro B, Arampatzis S, Manos B (2014) Public-private partnerships value in bioenergy projects: economic feasibility analysis based on two case studies. Biomass Bioenergy 66:387–397
Gezgin S, Dursun N, Hamurcu M, Harmankaya M, Onder M, Sade B, Topal A, Çiftçi N, Acar B, Babaoglu M (2002) Determination of boron contents of soils in Central-Anatolia cultivated lands and its relationship, between soil and water characteristics. In: Goldbah HE (ed) Boron in plant and animal nutrition, 1st edn. Kluwer Academic Publishers, New York, pp 391–400
González-Fontes A, Herrera-Rodriguez MB, Martín-Rejano EM, Navarro-Gochicoa MT, Rexach J, Camacho-Cristóbal JJ (2016) Root responses to boron deficiency mediated by ethylene. Front Plant Sci 6:1103
Gunes A, Soylemezoglu G, Inal A, Bagci EG, Coban S, Sahin O (2006) Antioxidant and stomatal responses of grapevine (Vitis vinifera L.) to boron toxicity. Sci Hortic 10:279–284
Guo T, Cibin R, Chaubey I, Gitau M, Arnold JG, Srinivasan R, Kiniry JR, Enge BA (2018) Evaluation of bioenergy crop growth and the impacts of bioenergy crops on stream flow, tile drain flow and nutrient losses in an extensively tile-drained watershed using SWAT. Sci Total Environ 613–614:724–735
Hakki EE, Atalay E, Harmankaya M, Babaoglu M, Hamurcu M, Gezgin S (2007) Determination ofsuitable maize (Zea mays L.) genotypes to be cultivated in boron-rich central anatolian soil. In: Xu F, Goldbach HE, Brown PH, Bell RW, Fujiwara T, Hunt CD (eds) Advances in plant and animal boron nutrition. Springer, Wuhan, pp 231–247
Halliday KJ, Martínez-García JF, Josse EM (2009) Integration of light and auxin signaling. Cold Spring Harb Perspect Biol 1:A001586
Hasan SA, Hayat S, Ahmad A (2011) Brassinosteroids protect photosynthetic machinery against the cadmium induced oxidative stress in two tomato cultivars. Chemosphere 84:1446–1451
Hayes JE, Reid RJ (2004) Boron tolerance in barley is mediated by efflux of boron from the roots. Plant Physiol 136(2):3376–3382
Jackson ML (1962) Soil chemical analysis. Prentice Hall, Englewood Cliffs, New York
Jezek M, Geilfus CM, Bayer A, Mühling K (2014) Photosynthetic capacity, nutrient status, and growth of maize (Zea mays L.) upon MgSO4 leaf-application. Front Plant Sci 5:781
Kaya C, Ashraf M (2015) Exogenous application of nitric oxide promotes growth and oxidative defense system in highly boron stressed tomato plants bearing fruit. Sci Hortic 185:43–47
Kaya C, Tuna AL, Dikilitas M, Ashraf M, Koskeroglu S, Guneri M (2009) Supplementary phosphorus can alleviate boron toxicity in tomato. Sci Hortic 121:284–288
Kraus TE, Fletcher RA (1994) Paclobutrazol protects wheat seedlings from heat and paraquat injury. Is detoxification of active oxygen involved? Plant Cell Physiol 35:45–52
Landi M, Pardossi A, Remorini D, Guidi L (2013) Antioxidant and photosynthetic response of a purple-leaved and a green-leaved cultivar of sweet basil (Ocimum basilicum) to boron excess. Environ Exp Bot 85:64–75
Latif M, Akram NA, Ashraf M (2016) Regulation of some biochemical attributes in drought-stressed cauliflower (Brassica oleracea L.) by seed pre-treatment with ascorbic acid. J Hortic Biotechnol 91(2):129–137
Leite VM, Rosolem CA, Rodrigues JD (2003) Gibberellin and cytokinin effects on soybean growth. Sci Agric 60:537–541
Li M, Guo R, Yu F, Chen X, Zhao H, Li H, Wu J (2018) Indole-3-acetic acid biosynthesis pathways in the plant-beneficial bacterium Arthrobacter pascens ZZ21. Int J Mol Sci 19:443
Loreto F, Velikova V (2001) Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiol 127:1781–1787
Ma HY (2007) Changes of endogenous hormones in grapevine during its development. Northwest Agriculture Forest University, Yangling
Macho-Rivero MA, Herrera-Rodríguez MB, Brejcha R, Schäffner AR, Tanaka N, Fujiwara T, Camacho-Cristóbal JJ (2018) Boron toxicity reduces water transport from root to shoot in Arabidopsis plants. Evidence for a reduced transpiration rate and expression of major PIP aquaporin genes. Plant Cell Physiol 59(4):836–844
Manos B, Partalidou M, Fantozzi F, Arampatzis S, Papadopoulou O (2014a) Agro-energy districts contributing to environmental and social sustainability in rural areas: evaluation of a local public-private partnership scheme in Greece. Renew Sustain Energy Rev 29:85–95
Manos B, Bartocci P, Partalidou M, Fantozzi F, Arampatzis S (2014b) Review of public-private partnerships in agro-energy districts in Southern Europe: the cases of Greece and Italy. Renew Sustain Energy Rev 39:667–678
Miwa K, Fujiwara T (2010) Boron transport in plants: co-ordinated regulation of transporters. Ann Bot 105(7):1103–1108
Molassiotis A, Sotiropoulos T, Tanou G, Diamantidis G, Therios I (2006) Boron induced oxidative damage and antioxidant and nucleolytic responses in shoot tips culture of the apple rootstock EM 9 (Malus domestica Borkh). Environ Exp Bot 55:54–62
Murchie EH, Lawson T (2013) Chlorophyll fluorescence analysis: a guide to good practice and understanding some new applications. J Exp Bot 64(13):3983–3998
Nable RO, Banuelou GS, Paull JG (1997) Boron toxicity. Plant Soil 198:111–115
Naz H, Akram NA, Ashraf M (2016) Impact of ascorbic acid on growth and some physiological attributes of cucumber (Cucumis sativus) plants under water-deficit conditions. Pak J Bot 48(3):877–883
Parks JL, Edwards M (2005) Boron in the environment. Crit Rev Env Sci Biotechnol 35:81–114
Patten CL, Glick BR (1996) Bacterial biosynthesis of indole-3-acetic acid. Can J Microbiol 42:207–220
Rashid N, Basra SMA, Shahbaz M, Iqbal S, Hafeez MB (2018) Foliar applied moringa leaf extract induces terminal heat tolerance in quinoa. Int J Agric Biol 20:157–164
Reid R (2007) Identification of boron transporter genes likely to be responsible for tolerance to boron toxicity in wheat and barley. Plant Cell Physiol 48(12):1673–1678
Reid RJ (2013) Boron toxicity and tolerance in crop plants. In: Crop improvement under adverse conditions. Springer, New York, pp 333–346
Rivero RM, Gimeno J, Deynze AV, Walia H, Blumwald E (2010) Enhanced cytokinin synthesis in tobacco plants expressing PSARK: IPT prevents the degradation of photosynthetic protein complexes during drought. Plant Cell Physiol 55:1929–1941
Rubio V, Bustos R, Irigoyen ML, Cardona-López X, Rojas-Triana M, Paz-Ares J (2009) Plant hormones and nutrient signaling. Plant Mol Biol 69(4):361
Sarwat M, Naqvi AR, Ahmad P, Ashraf M, Akram NA (2013) Phytohormones and micro RNAs as sensors and regulators of leaf senescence: assigning macro roles to small molecules. Biotechnol Adv 31(8):1153–1171
Schmucker T (1933) Zur Blütenbiologie tropischer Nymphaea-Arten II (Bor als entscheidender Faktor.). Planta 18(4):641–650
Sekhon RS, Breitzman MW, Silva RR, Santoro N, Rooney WL, de Leon N et al (2016) Stover composition in maize and sorghum reveals remarkable genetic variation and plasticity for carbohydrate accumulation. Front Plant Sci. https://doi.org/10.3389/fpls.2016.00822
Sessitsch A, Reiter B, Berg G (2004) Endophytic bacterial communities of field-grown potato plants and their plant-growth-promoting and antagonistic abilities. Can J Microbiol 50:239–249
Spaepen S, Vanderleyden J, Remans R (2007) Indole-3-acetic acid in microbial and microorganism-plant signaling. FEMS Microbiol Rev 31:425–448
Tavallali V (2017) Interactive effects of zinc and boron on growth, photosynthesis, and water relations in pistachio. J Plant Nutr 40(11):1588–1603
Tran LSP, Urao T, Qin F, Maruyama K, Kakimoto T, Shinozaki K, Yamaguchi-Shinozaki K (2007) Functional analysis of AHK1/ATHK1 and cytokinin receptor histidine kinases in response toabscisic acid, drought, and salt stress in Arabidopsis. Proc Natl Acad Sci USA 104:20623–20628
Upreti KK, Sharma M (2016) Role of plant growth regulators in abiotic stress tolerance. In: Rao NKS (eds) Abiotic stress physiology of horticultural crops. Springer, New York https://doi.org/10.1007/978-81-322-2725-0_2
Vankova R, Gaudinova A, Dobrev P, Malbeck J, Haisel D, Motyka V (2011) Comparison of salinity and drought stress effects on abscisic acid metabolites activity of cytokinin oxidase/dehydrogenase and chlorophyll levels in radish and tobacco. Ecol Quest 14:99–100
Vasudevan PT, Briggs M (2008) Biodiesel production: current state of the art and challenges. J Ind Microbiol Biotechnol 35:421–430
Weatherley PE, Barrs C (1962) A re-examination of the relative turgidity technique forestimating water deficits in leaves. Aust J Biol Sci 5:413–428
Weisany W, Sohrabi Y, Heidari G, Siosemardeh A, Ghassemi-Golezani K (2012) Changes in antioxidant enzymes activity and plant performance by salinity stress andzinc application in soybean (Glycine max L.). Plant Omics J 5:60–67
Wimmer MA, Eichert T (2013) Mechanisms for boron deficiency-mediated changes in plant water relations. Plant Sci 203:25–32
Wolf B (1971) The determination of boron in soil extracts, plant materials, composts,manures, water and nutrient solutions. Commun Soil Sci Plant Anal 2:363–374
You J, Chan Z (2015) ROS regulation during abiotic stress responses in crop plants. Front Plant Sci 6:1092
Yusuf M, Fariduddin Q, Ahmad A (2011) 28-Homobrassinolide mitigates boron induced toxicity through enhanced antioxidant system Vigna radiata plants. Chemosphere 85:1574–1584
Zhao Y (2010) Auxin biosynthesis and its role in plant development. Ann Rev Plant Biol 61:49–64
Acknowledgements
The authors wish to thank the University of Harran (Turkey) and GC University, Faisalabad (Pakistan) for supporting the present study.
Author information
Authors and Affiliations
Contributions
CK and NAA conducted the experimentation and data analysis, respectively. Both also jointly wrote up the manuscript. MA helped in designing the study and edited critically the whole manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest regarding the publication of this paper.
Rights and permissions
About this article
Cite this article
Kaya, C., Akram, N.A. & Ashraf, M. Kinetin and Indole Acetic Acid Promote Antioxidant Defense System and Reduce Oxidative Stress in Maize (Zea mays L.) Plants Grown at Boron Toxicity. J Plant Growth Regul 37, 1258–1266 (2018). https://doi.org/10.1007/s00344-018-9827-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-018-9827-6