Abstract
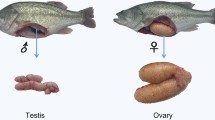
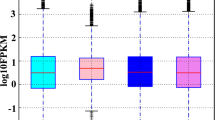
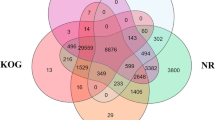
Mandarin fish (Siniperca chuatsi) is an economically important freshwater fish cultured in China. In this species, females grow faster than males. However, due to the lack of available genomic and transcriptome information, the mechanisms of sex differentiation remain poorly understood. In this study, Illumina high-throughput sequencing technology was used to sequence four cDNA libraries; the tissues examined included hypothalamus, pituitary gland, ovary, and testis. A total of 134 124 high-quality unigenes were obtained on average length of 1 361 bp and N50 of 3 312 bp. A search of all-unigene sequences against NR, NT, SwissProt, KOG, KEGG, GO, and InterPro databases resulted in 59 688 (44.50%), 76 329 (56.91%), 50 432 (37.60%), 45 741 (34.10%), 48 760 (36.35%), 5 241 (3.91%), and 46 099 (34.37%) annotations, respectively. In a comparison of ovarian and testicular libraries, 15 289 ovary-biased genes and 10 035 testis-biased genes were identified, including a series of genes related to sex determination and differentiation, such as cyp19a1a, foxl2, sox9, dmrtl, amh, and others. In addition, 49 495 SSRs and 85 899 SNPs were detected in transcriptome data. Quantitative real-time PCR results of 15 sex-related functional genes indicated that RNA-seq data was reliable. This study will contribute to a better understanding of the molecular mechanisms of sex differentiation and development in Mandarin fish.
Similar content being viewed by others
Data Availability Statement
All raw reads of transcriptome sequencing data have been deposited at the NCBI Short Read Archive (SRA) database (SRA accession Nos.: SRR11743000, SRR11743001, SRR11743002, SRR11743003).
References
Chen S L, Zhang G J, Shao C W, Huang Q F, Liu G, Zhang P, Song W T, An N, Chalopin D, Volff J N, Hong Y H, Li Q Y, Sha Z X, Zhou H L, Xie M S, Yu Q L, Liu Y, Xiang H, Wang N, Wu K, Yang C G, Zhou Q, Liao X L, Yang L F, Hu Q M, Zhang J L, Meng L, Jin L J, Tian Y S, Lian J M, Yang J F, Miao G D, Liu S S, Liang Z, Yan F, Li Y Z, Sun B, Zhang H, Zhang J, Zhu Y, Du M, Zhao Y W, Schartl M, Tang Q S, Wang J. 2014. Whole-genome sequence of a flatfish provides insights into ZW sex chromosome evolution and adaptation to a benthic lifestyle. Nature Genetics, 46(3): 253–260, https://doi.org/10.1038/ng.2890.
Chu W Y, Fu G H, Chen J, Chen D G, Meng T, Zhou R X, Xia X J, Zhang J S. 2010. Gene expression profiling in muscle tissues of the commercially important teleost, Siniperca chuatsi L. Aquaculture International, 18(4): 667–678, https://doi.org/10.1007/s10499-009-9289-8.
Du X X, Wang B, Liu X M, Liu X B, He Y, Zhang Q Q, Wang X B. 2017. Comparative transcriptome analysis of ovary and testis reveals potential sex-related genes and pathways in spotted knifejaw Oplegnathus punctatus. Gene, 637: 203–210, https://doi.org/10.1016/j.gene.2017.09.055.
Fan Z F, You F, Wang L J, Weng S D, Wu Z H, Hu J W, Zou Y X, Tan X G, Zhang P J. 2014. Gonadal transcriptome analysis of male and female olive flounder (Paralichthys olivaceus). BioMed Research International, 2014: 291067, https://doi.org/10.1155/2014/291067.
Froese R, Pauly D. 2017. FishBase. World Wide Web electronic publication. www.fishbase.org. Accessed on 2020-5-1.
Georges A, Auguste A, Bessière L, Vanet A, Todeschini A L, Veitia R A. 2014. FOXL2: a central transcription factor of the ovary. Journal of Molecular Endocrinology, 52(1): R17–R33, https://doi.org/10.1530/JME-13-0159.
Guiguen Y, Fostier A, Piferrer F, Chang C F. 2010. Ovarian aromatase and estrogens: a pivotal role for gonadal sex differentiation and sex change in fish. General and Comparative Endocrinology, 165(3): 352–366, https://doi.org/10.1016/j.ygcen.2009.03.002.
Hagihara S, Yamashita R, Yamamoto S, Ishihara M, Abe T, Ijiri S, Adachi S. 2014. Identification of genes involved in gonadal sex differentiation and the dimorphic expression pattern in undifferentiated gonads of Russian sturgeon Acipenser gueldenstaedtii Brandt & Ratzeburg, 1833. Journal of Applied Ichthyology, 30(6): 1 557–1 564, https://doi.org/10.1111/jai.12588.
Hattori R S, Murai Y, Oura M, Masuda S, Majhi S K, Sakamoto T, Fernandino J I, Somoza G M, Yokota M, Strüssmann C A. 2012. A Y-linked anti-Müllerian hormone duplication takes over a critical role in sex determination. Proceedings of National Academy of Sciences of the United States of America, 109(8): 2 955–2 959, https://doi.org/10.1073/pnas.1018392109.
He S, Liang X F, Sun J, Li L, Yu Y, Huang W, Qu C M, Cao L, Bai X L, Tao Y X. 2013. Insights into food preference in hybrid F1 of Siniperca chuatsi (♀) x Siniperca scherzeri (♂) mandarin fish through transcriptome analysis. BMC Genomics, 14: 601, https://doi.org/10.1186/1471-2164.14-601.
He Y H, Li L, Liang X F, He S, Zhao L, Zhang Y P. 2018. Inhibitory neurotransmitter serotonin and excitatory neurotransmitter dopamine both decrease food intake in Chinese perch (Siniperca chuatsi). Fish Physiology and Biochemistry, 44(1): 175–183, https://doi.org/10.1007/s10695-017-0422-8.
Hildahl J, Sandvik G K, Edvardsen R B, Fagernes C, Norberg B, Haug T M, Weltzien F A. 2011. Identification and gene expression analysis of three GnRH genes in female Atlantic cod during puberty provides insight into GnRH variant gene loss in fish. General and Comparative Endocrinology, 172(3): 458–467, https://doi.org/10.1016/j.ygcen.2011.04.010.
Ikemoto T, Park M K. 2005. Identification and molecular characterization of three GnRH ligands and five GnRH receptors in the spotted green pufferfish. Molecular and Cellular Endocrinology, 242(1–2): 67–79, https://doi.org/10.1016/j.mce.2005.07.004.
Janzen F J. 1995. Experimental evidence for the evolutionary significance of temperature-dependent sex determination. Evolution, 49(5): 864–873, https://doi.org/10.1111/j.1558-5646.1995.tb02322.x.
Kamiya T, Kai W, Tasumi S, Oka A, Matsunaga T, Mizuno N, Fujita M, Suetake H, Suzuki S, Hosoya S, Tohari S, Brenner S, Miyadai T, Venkatesh B, Suzuki Y, Kikuchi K. 2012. A trans-species missense SNP in Amhr2 is associated with sex determination in the tiger pufferfish, Takifugu rubripes (fugu). PLoS Genetics, 8(7): e1002798, https://doi.org/10.1371/journal.pgen.1002798.
Lethimonier C, Madigou T, Muñoz-Cueto J A, Lareyre J J, Kah O. 2004. Evolutionary aspects of GnRHs, GnRH neuronal systems and GnRH receptors in teleost fish. General and Comparative Endocrinology, 135(1): 1–16, https://doi.org/10.1016/j.ygcen.2003.10.007.
Li M H, Sun L, Wang D S. 2019a. Roles of estrogens in fish sexual plasticity and sex differentiation. General and Comparative Endocrinology, 277: 9–16, https://doi.org/10.1016/j.ygcen.2018.11.015.
Li M H, Sun Y L, Zhao J, Shi H J, Zeng S, Ye K, Jiang D N, Zhou L Y, Sun L N, Tao W J, Nagahama Y, Kocher T D, Wang D S. 2015. A tandem duplicate of anti-müllerian hormone with a missense SNP on the Y chromosome is essential for male sex determination in Nile Tilapia, Oreochromis niloticus. PLoS Genetics, 11(11): e1005678, https://doi.org/10.1371/journal.pgen.1005678.
Li M H, Yang H H, Li M R, Sun Y L, Jiang X L, Xie Q P, Wang T R, Shi H J, Sun L N, Zhou L Y, Wang D S. 2013. Antagonistic roles of Dmrt1 and Foxl2 in sex differentiation via estrogen production in tilapia as demonstrated by TALENs. Endocrinology, 154(12): 4 814–4 825, https://doi.org/10.1210/en.2013-1451.
Li Z J, Guo R T, Gu Z Z, Wang X N, Wang Y C, Xu H F, Wang C X, Liu X J. 2019b. Identification of a promoter element mediating kisspeptin-induced increases in GnRH gene expression in sheep. Gene, 699: 1–7, https://doi.org/10.1016/j.gene.2019.03.006.
Liang X F, Kiu J K, Huang B Y. 1998. The role of sense organs in the feeding behaviour of Chinese perch. Journal of Fish Biology, 52(5): 1 058–1 067, https://doi.org/10.1111/j.1095-8649.1998.tb00603.x.
Lindeman R E, Gearhart M D, Minkina A, Krentz A D, Bardwell V J, Zarkower D. 2015. Sexual cell-fate reprogramming in the ovary by DMRT1. Current Biology, 25(6): 764–771, https://doi.org/10.1016/j.cub.2015.01.034.
Lu S Q, Liu F, Liu Z, Zhang J S, Xie X M. 2008. Comparison in cloning and sequence of growth hormone gene in three species of genus Siniperca. Oceanologia et Limnologia Sinica, 39(4): 354–361. (in Chinese with English abstract)
Matsuda M, Nagahama Y, Shinomiya A, Sato T, Matsuda C, Kobayashi T, Morrey C E, Shibata N, Asakawa S, Shimizu N, Hori H, Hamaguchi S, Sakaizumi M. 2002. DMY is a Y-specific DM-domain gene required for male development in the medaka fish. Nature, 417(6888): 559–563, https://doi.org/10.1038/nature751.
Mechaly A S, Viñas J, Piferrer F. 2011. Gene structure analysis of kisspeptin-2 (Kiss2) in the Senegalese sole (Solea senegalensis): characterization of two splice variants of Kiss2, and novel evidence for metabolic regulation of kisspeptin signaling in non-mammalian species. Molecular and Cellular Endocrinology, 339(1–2): 14–24, https://doi.org/10.1016/j.mce.2011.03.004.
Miller W L. 2007. Steroidogenic acute regulatory protein (StAR), a novel mitochondrial cholesterol transporter. Biochimica et Biophysica Acta (BBA) — Molecular and Cell Biology of Lipids, 1771(6): 663–676, https://doi.org/10.1016/j.bbalip.2007.02.012.
Moncaut N, Somoza G, Power D M, Canario A V M. 2005. Five gonadotrophin-releasing hormone receptors in a teleost fish: isolation, tissue distribution and phylogenetic relationships. Journal of Molecular Endocrinology, 34(3): 767–779, https://doi.org/10.1677/jme.L01757.
Myosho T, Otake H, Masuyama H, Matsuda M, Kuroki Y, Fujiyama A, Naruse K, Hamaguchi S, Sakaizumi M. 2012. Tracing the emergence of a novel sex-determining gene in medaka, Oryzias luzonensis. Genetics, 191(1): 163–170, https://doi.org/10.1534/genetics.111.137497.
Nanda I, Kondo M, Hornung U, Asakawa S, Winkler C, Shimizu A, Shan Z H, Haaf T, Shimizu N, Shima A, Schmid M, Schartl M. 2002. A duplicated copy of DMRT1 in the sex-determining region of the Y chromosome of the medaka, Oryzias latipes. Proceedings of the National Academy of Sciences of the United States of America, 99(18): 11 778–11 783, https://doi.org/10.1073/pnas.182314699.
Nef S, Vassalli J D. 2009. Complementary pathways in mammalian female sex determination. Journal of Biology, 8(8): 74, https://doi.org/10.1186/jbiol173.
Novaira H J, Ng Y, Wolfe A, Radovick S. 2009. Kisspeptin increases GnRH mRNA expression and secretion in GnRH secreting neuronal cell lines. Molecular and Cellular Endocrinology, 311(1–2): 126–134, https://doi.org/10.1016/j.mce.2009.06.011.
Ogino Y, Tohyama S, Kohno S, Toyota K, Yamada G, Yatsu R, Kobayashi T, Tatarazako N, Sato T, Matsubara H, Lange A, Tyler C R, Katsu Y, Iguchi T, Miyagawa S. 2018. Functional distinctions associated with the diversity of sex steroid hormone receptors ESR and AR. The Journal of Steroid Biochemistry and Molecular Biology, 184: 38–46, https://doi.org/10.1016/j.jsbmb.2018.06.002.
Ospina-Álvarez N, Piferrer F. 2008. Temperature-dependent sex determination in fish revisited: prevalence, a single sex ratio response pattern, and possible effects of climate change. PLoS One, 3(7): e2837, https://doi.org/10.1371/journal.pone.0002837.
Reinhart A J, Williams S C, Stocco D M. 1999. Transcriptional regulation of the StAR gene. Molecular and Cellular Endocrinology, 151(1–2): 161–169, https://doi.org/10.1016/S0303-7207(98)00257-3.
Robinson-Rechavi M, Laudet V. 2001. Evolutionary rates of duplicate genes in fish and mammals. Molecular Biology and Evolution, 18(4): 681–683, https://doi.org/10.1093/oxfordjournals.molbev.a003849.
Robinson-Rechavi M, Marchand O, Escriva H, Bardet P L, Zelus D, Hughes S, Laudet V. 2001. Euteleost fish genomes are characterized by expansion of gene families. Genome Research, 11(5): 781–788, https://doi.org/10.1101/gr.165601.
Rodríguez-Marí A, Yan Y L, Bremiller R A, Wilson C, Cañestro C, Postlethwait J H. 2005. Characterization and expression pattern of zebrafish anti-Müllerian hormone (amh) relative to sox9a, sox9b, and cyp19a1a, during gonad development. Gene Expression Patterns, 5(5): 655–667, https://doi.org/10.1016/j.modgep.2005.02.008.
Rouiller-Fabre V, Carmona S, Merhi R A, Cate R, Habert R, Vigier B. 1998. Effect of anti-Mullerian hormone on sertoli and leydig cell functions in fetal and immature rats. Endocrinology, 139(3): 1 213–1 220, https://doi.org/10.1210/endo.139.3.5785.
Schartl M, Wilde B, Schlupp I, Parzefall J. 1995. Evolutionary origin of a parthenoform, the Amazon molly Poecilia formosa, on the basis of a molecular genealogy. Evolution, 49(5): 827–835, https://doi.org/10.2307/2410406.
Selstam G, Rosberg S, Liljekvist J, Grönquist L, Perklev T, Ahrén K. 1976. Differences in action of LH and FSH on the formation of cyclic amp in the prepubertal rat ovary. European Journal of Endocrinology, 81(1): 150–164, https://doi.org/10.1530/acta.0.0810150.
Selvaraj S, Kitano H, Fujinaga Y, Ohga H, Yoneda M, Yamaguchi A, Shimizu A, Matsuyama M. 2010. Molecular characterization, tissue distribution, and mRNA expression profiles of two Kiss genes in the adult male and female chub mackerel (Scomber japonicus) during different gonadal stages. General and Comparative Endocrinology, 169(1): 28–38, https://doi.org/10.1016/).ygcen.2010.07.011.
Shi B Y, Lu H J, Zhang L H, Zhang W M. 2019. Nr5a1b promotes and Nr5a2 inhibits transcription of lhb in the orange-spotted grouper, Epinephelus coioides. Biology of Reproduction, 101(4): 800–812, https://doi.org/10.1093/biolre/ioz121.
Simpson E R, Mahendroo M S, Means G D, Kilgore M W, Hinshelwood M M, Graham-Lorence S, Amarneh B, Ito Y, Fisher C R, Michael M D, Mendelson C R, Bulun S E. 1994. Aromatase cytochrome P450, the enzyme responsible for estrogen biosynthesis. Endocrine Reviews, 15(3): 342–355, https://doi.org/10.1210/er.15.3.342.
Sreenivasan R, Cai M, Bartfai R, Wang X, Christoffels A, Orban L. 2008. Transcriptomic analyses reveal novel genes with sexually dimorphic expression in the zebrafish gonad and brain. PLoS One, 3(3): e1791, https://doi.org/10.1371/journal.pone.0001791.
Stocco D M, Wang X J, Jo Y, Manna P R. 2005. Multiple signaling pathways regulating steroidogenesis and steroidogenic acute regulatory protein expression: more complicated than we thought. Molecular Endocrinology, 19(11): 2 647–2 659, https://doi.org/10.1210/me.2004-0532.
Sun F, Liu S K, Gao X Y, Jiang Y L, Perera D, Wang X L, Li C, Sun L Y, Zhang J R, Kaltenboeck L, Dunham R, Liu Z J. 2013. Male-biased genes in catfish as revealed by RNA, Seq analysis of the testis transcriptome. PLoS One, 8(7): e68452, https://doi.org/10.1371/journal.pone.0068452.
Tao W J, Yuan J, Zhou L Y, Sun L N, Sun Y L, Yang S J, Li M H, Zeng S, Huang B F, Wang D S. 2013. Characterization of gonadal transcriptomes from Nile tilapia (Oreochromis niloticus) reveals differentially expressed genes. PLoS One, 8(5): e63604, https://doi.org/10.1371/journal.pone.0063604.
Tu J G, Tian C X, Zhao P Q, Sun J X, Wang M, Fan Q X, Yuan Y C. 2017. Identification and profiling of growth-related microRNAs in Chinese perch (Siniperca chuatsi). BMC Genomics, 18(1): 489, https://doi.org/10.1186/s12864.017-3851-y.
Wang D S, Kobayashi T, Zhou L Y, Paul-Prasanth B, Fumie S, Sakai F, Okubo K, Morohashi K, Nagahama Y. 2007. Foxl2 up-regulates aromatase gene transcription in a female-specific manner by binding to the promoter as well as interacting with ad4 binding protein/steroidogenic factor 1. Molecular Endocrinology, 21(3): 712–725, https://doi.org/10.1210/me.2006-0248.
Wang D S, Zhou L Y, Kobayashi T, Matsuda M, Shibata Y, Sakai F, Nagahama Y. 2010. Doublesex, and Mab-3-related transcription factor-1 repression of aromatase transcription, a possible mechanism favoring the male pathway in tilapia. Endocrinology, 151(3): 1 331–1 340, https://doi.org/10.1210/en.2009-0999.
Wang G L, Wang M C, Zhang X W, Chang M X, Xie H X, Nie P. 2017a. Molecular cloning, biological effect, and tissue distribution of interleukin-8 protein in mandarin fish (Siniperca chuasti) upon Flavobacterium columnare infection. Fish & Shellfish Immunology, 66: 112–119, https://doi.org/10.1016/j.fsi.2017.05.016.
Wang G, Li J H, Zou P F, Xie H X, Huang B, Nie P, Chang M X. 2012. Expression pattern, promoter activity and bactericidal property of β-defensin from the mandarin fish Siniperca chuatsi. Fish & Shellfish Immunology, 33(3): 522–531, https://doi.org/10.1016/j.fsi.2012.06.003.
Wang K Z, Zhu X, Li Y L, Chen D X, Wu P, Chu W Y. 2016. Molecular characterization and expression regulation of Smyd1a and Smyd1b in skeletal muscle of Chinese perch (Siniperca chuatsi). Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 194-195: 25–31, https://doi.org/10.1016/j.cbpb.2016.01.004.
Wang P F, Zeng S, Xu P, Zhou L, Zeng L, Lu X, Wang H F, Li G F. 2014. Identification and expression analysis of two HSP70 isoforms in mandarin fish Siniperca chuatsi. Fisheries Science, 80(4): 803–817, https://doi.org/10.1007/s12562-014-0747-5.
Wang X Q, Li C W, XIE Z G, Fan W J, Zhang J S. 2006. Studies on the growth difference of the male and female Siniperca chuatsi. Freshwater Fisheries, 36(3): 34–37. (in Chinese with English abstract)
Wang Z C, Qiu X M, Kong D, Zhou X X, Guo Z B, Gao C F, Ma S, Hao W W, Jiang Z Q, Liu S C, Zhang T, Meng X S, Wang X L. 2017b. Comparative RNA-Seq analysis of differentially expressed genes in the testis and ovary of Takifugu rubripes. Comparative Biochemistry and Physiology Part D: Genomics and Proteomics, 22: 50–57, https://doi.org/10.1016/j.cbd.2017.02.002.
Warner R R. 1984. Mating behavior and hermaphroditism in coral reef fishes: the diverse forms of sexuality found among tropical marine fishes can be viewed as adaptations to their equally diverse mating systems. American Scientist, 72(2): 128–136.
Webster K A, Schach U, Ordaz A, Steinfeld J S, Draper B W, Siegfried K R. 2017. Dmrt1 is necessary for male sexual development in zebrafish. Developmental Biology, 422(1): 33–46, https://doi.org/10.1016/j.ydbio.2016.12.008.
Wei L, Li X Y, Li M H, Tang Y H, Wei J, Wang D S. 2019. Dmrt1 directly regulates the transcription of the testis-biased Sox9b gene in Nile tilapia (Oreochromis niloticus). Gene, 687: 109–115, https://doi.org/10.1016/j.gene.2018.11.016.
Weltzien F A, Andersson E, Andersen Ø, Shalchian-Tabrizi K, Norberg B. 2004. The brain-pituitary-gonad axis in male teleosts, with special emphasis on flatfish (Pleuronectiformes). Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 137(3): 447–477, https://doi.org/10.1016/j.cbpb.2003.11.007.
Yamaguchi T, Yamaguchi S, Hirai T, Kitano T. 2007. Follicle-stimulating hormone signaling and Foxl2 are involved in transcriptional regulation of aromatase gene during gonadal sex differentiation in Japanese flounder, Paralichthys olivaceus. Biochemical and Biophysical Research Communications, 359(4): 935–940, https://doi.org/10.1016/j.bbrc.2007.05.208.
Yano A, Guyomard R, Nicol B, Jouanno E, Quillet E, Klopp C, Cabau C, Bouchez O, Fostier A, Guiguen Y. 2012. An immune-related gene evolved into the master sex, determining gene in rainbow trout, Oncorhynchus mykiss. Current Biology, 22(15): 1 423–1 428, https://doi.org/10.1016/j.cub.2012.05.045.
Zhang G Q, Chu W Y, Hu S N, Meng T, Pan L L, Zhou R X, Liu Z, Zhang J S. 2011. Identification and analysis of muscle-related protein isoforms expressed in the white muscle of the mandarin fish (Siniperca chuatsi). Marine Biotechnology, 13(2): 151–162, https://doi.org/10.1007/s10126-010-9275-1.
Zou L J, Gong J, Ji L, Zhuang Y H, Liao H Y, Huang W. 2017. Cloning and expression analysis of CYP19a gene in mandarin fish Siniperca chuatsi. Life Science Research, 21(4): 295–301. (in Chinese with English abstract)
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the National Key R&D Program of China (No. 2018YFD0901203), the Guangdong Basic and Applied Basic Research Foundation (No. 2019B1515120072), and the Science and Technology Planning Project of Guangzhou (No. 201904020043)
Rights and permissions
About this article
Cite this article
Zhu, Q., Han, C., Peng, C. et al. Identification of potential sex-related genes in Siniperca chuatsi. J. Ocean. Limnol. 39, 1500–1512 (2021). https://doi.org/10.1007/s00343-020-0251-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00343-020-0251-y