Abstract
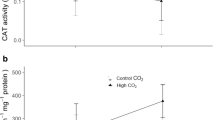
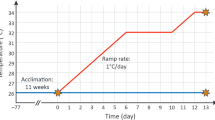
Global impacts are affecting negatively coral reefs’ health worldwide. Ocean acidification associated with the increasing CO2 partial pressure in the atmosphere can potentially induce oxidative stress with consequent cellular damage in corals and hydrocorals. In the present study, parameters related to oxidative status were evaluated in the hydrocoral Millepora alcicornis exposed to three different levels of seawater acidification using a mesocosm system. CO2-driven acidification of seawater was performed until reaching 0.3, 0.6 and 0.9 pH units below the current pH of seawater pumped from the coral reef adjacent to the mesocosm. Therefore, treatments corresponded to control (pH 8.1), mild (pH 7.8), intermediate (pH 7.5) and severe (pH 7.2) seawater acidification. After 0, 16 and 30 d of exposure, hydrocorals were collected and the following parameters were analyzed in the holobiont: antioxidant capacity against peroxyl radicals (ACAP), total glutathione (GSHt) concentration, reduced (GSH) and oxidized (GSSG) glutathione ratio (GSH/GSSG), lipid peroxidation (LPO) and protein carbonyl group (PC) levels. ACAP was increased in hydrocorals after 16 d of exposure to intermediate levels of seawater acidification. GSHt and GSH/GSSG did not change over the experimental period. LPO was increased at any level of seawater acidification, while PC content was increased in hydrocorals exposed to intermediate and severe seawater acidification for 30 d. These findings indicate that the antioxidant defense system of M. alcicornis is capable of coping with acidic conditions for a short period of time (16 d). Additionally, they clearly show that a long-term (30 d) exposure to seawater acidification induces oxidative stress with consequent oxidative damage to lipids and proteins, which could compromise hydrocoral health.






Similar content being viewed by others
References
Abele D, Vazquez-Medina JP, Zenteno-Savin T (2012) Oxidative Stress in Aquatic Ecosystems. Wiley-Blackwell, Hoboken
Amado LL, Garcia ML, Ramos PB, Freitas RF, Zafalon B, Ferreira JLR, Yunes JS, Monserrat JM (2009) A method to measure total antioxidant capacity against peroxyl radicals in aquatic organisms: Application to evaluate microcystins toxicity. Sci Total Environ 407:2115–2123
Anthony K, Connolly SR, Hoegh-Guldberg O (2007) Bleaching, energetics, and coral mortality risk: Effects of temperature, light, and sediment regime. Limnol Oceanogr 52:716–726
Brown D, Edmunds PJ (2016) Differences in the responses of three scleractinians and the hydrocoral Millepora platyphylla to ocean acidification. Mar Biol 163:62
Cohen AL, Holcomb M (2009) Why corals care about ocean acidification: uncovering the mechanism. Oceanography 22:118–127
Cohen AL, McCorkle DC, de Putron S, Gaetani GA, Rose KA (2009) Morphological and compositional changes in the skeletons of new coral recruits reared in acidified seawater: Insights into the biomineralization response to ocean acidification. Geochem Geophys Geosystems 10:1–12
Costa V, Quintanilha A, Moradas-Ferreira P (2007) Protein oxidation, repair mechanisms and proteolysis in Saccharomyces cerevisiae. IUBMB Life 59:293–298
Dalvi SM, Patil VW, Ramraje NN, Phadtare JM, Gujarathi SU (2013) Nitric oxide, carbonyl protein, lipid peroxidation and correlation between antioxidant vitamins in different categories of pulmonary and extra pulmonary tuberculosis. Malays J Med Sci 20:21–30
Downs CA (2005) Cellular diagnostics and its application to aquatic and marine toxicology. In: Ostrander GK (ed) Techniques in Aquatic Toxicology. CRC Press, Boca Raton, pp 101–208
Downs CA, Fauth JE, Halas JC, Dustan P, Bemiss J, Woodley CM (2002) Oxidative stress and seasonal coral bleaching. Free Rad Biol Med 33:533–543
Duarte G, Calderon EN, Pereira CM, Marangoni LFB, Santos HF, Peixoto RS, Bianchini A, Castro CB (2015) A novel marine mesocosm facility to study global warming, water quality, and ocean acidification. Ecol Evol 5:4555–4566
Field CB, Barros VR, Dokken DJ, Mastrandrea MD, Bilir TE, Chatterjee M, Ebi KL, Estrada YO, Genova RC, Girma B et al (2014) Climate change 2014: impacts, adaptation, and vulnerability. Cambridge University Press, New York, Part A
Freitas R, Pires A, Moreira A, Wrona FJ, Figueira E, Soares AMVM (2016) Biochemical alterations induced in Hediste diversicolor under seawater acidification conditions. Mar Environ Res 117:75–84
Gates RD, Baghdasarian G, Muscatine L (1992) Temperature stress causes host cell detachment in symbiotic cnidarians: Implications for coral bleaching. Biol Bull 182:324–332
Gattuso J-P, Hansson L (2011) Ocean acidification. Oxford University Press, New York
Halpern BS, Selkoe KA, Micheli F, Kappel CV (2007) Evaluating and ranking the vulnerability of global marine ecosystems to anthropogenic threats. Conserv Biol 21:1301–1315
Hennige S, Roberts JM, Williamson P (2014) An Updated Synthesis of the Impacts of Ocean Acidification on Marine Biodiversity. Technical Series No. 75, Secretariat of the Convention on Biological Diversity, Montreal
Hermes-Lima M (2004) Oxygen in biology and biochemistry: Role of free radicals. In: Storey KB (ed) Functional Metabolism Regulation and Adaptation. Wiley, Hoboken, pp 319–368
IPCC (2014) Climate Change 2014 – Impacts, Adaptation and Vulnerability: Part A: Global and Sectoral Aspects. Cambridge University Press, Cambridge
Kaniewska P, Campbell PR, Kline DI, Rodriguez-Lanetty M, Miller DJ, Dove S, Hoegh-Guldberg O (2012) Major cellular and physiological impacts of ocean acidification on a reef building coral. PloS one 7:e34659
Kulkarni A, Madrasi NA (2008) Relationship of nitric oxide and protein carbonyl in tuberculosis. Indian J Tuberc 55:138–144
Lamsal M, Narayan G (2007) Evaluation of lipid peroxidation product, nitrite and antioxidant levels in newly diagnosed and two months follow-up patients with pulmonary tuberculosis. Southeast Asian J Trop Med Public Health 38:695–703
Leal ICS, Pereira PHC, Araújo ME (2013) Coral reef fish association and behaviour on the fire coral Millepora spp. in north-east Brazil. J Mar Biol Assoc UK 93:1703–1711
Leal ICS, Araújo ME, Cunha SR, Pereira PHC (2015) The influence of fire-coral colony size and agonistic behaviour of territorial damselfish on associated coral reef fish communities. Mar Environ Res 108:45–54
Leão ZMAN, Kikuchi RKP, Testa V (2003) Corals and coral reefs of Brazil. In: Cortés J (ed) Latin American Coral Reefs. Elsevier, Amsterdan, pp 9–52
Levine RL, Garland D (1990) Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol 186:464–478
Lewis JB (2006) Biology and ecology of the hydrocoral Millepora on coral reefs. Adv Mar Biol 50:1–55
Marangoni LFB, Calderon EN, Marques JA, Duarte GAS, Pereira CM, Castro CB, Bianchini A (2017) Effects of CO2-driven acidification of seawater on the calcification process in the calcareous hydrozoan Millepora alcicornis (Linnaeus, 1758). Coral reefs 36:1133–1141
Mohod K, Dhok A, Kumar S (2011) Status of oxidants and antioxidants in pulmonary tuberculosis with varying bacillary load. J Exp Sci. 2:35–37
Oakes KD, Van Der Kraak GJ (2003) Utility of the TBARS assay in detecting oxidative stress in white sucker (Catostomus commersoni) populations exposed to pulp mill effluent. Aquat Toxicol 63:447–463
Pereira PHC, Leal ICS, Araújo ME, Souza AT (2012) Feeding association between reef fishes and the fire coral Millepora spp. (Cnidaria: Hydrozoa). Mar Biod Rec 5:1–4
Pörtner H-O, Gutowska M, Ishimatsu A, Lucassen M, Melzner F, Seibel B (2011) Effects of ocean acidification on nektonic organisms. In: Gattuso J-P, Hanson L (eds) ocean acidification. Oxford University Press, New York, pp 154–175
Sarmento VC, Souza TP, Esteves AM, Santos PJP (2015) Effects of seawater acidification on a coral reef meiofauna community. Coral Reefs 34:955–966
Siddiqui S, Bielmyer-Fraser GK (2015) Responses of the sea anemone, Exaiptasia pallida, to ocean acidification conditions and copper exposure. Aquat Toxicol 167:228–239
Soriano-Santiago OS, Liñán-Cabello MA, Delgadillo-Nuño MA, Ortega-Ortiz C, Cuevas-Venegas S (2013) Physiological responses to oxidative stress associated with pH variations in host tissue and zooxanthellae of hermatypic coral Pocillopora capitata. Mar Fresh Behav Physiol 46:275–286
Strahl J, Francis DS, Doyle J, Humphrey C, Fabricius KE (2015) Biochemical responses to ocean acidification contrast between tropical corals with high and low abundances at volcanic carbon dioxide seeps. ICES J Mar Sci 73:897–909
Wäge J, Lerebours A, Hardege JD, Rotchell JM (2016) Exposure to low pH induces molecular level changes in the marine worm, Platynereis dumerilii. Ecotoxicol Environ Saf 124:105–110
Acknowledgements
The International Development Research Centre (IDRC, Ottawa, Canada; Grant No. 104519-003), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES—Programa Ciências do Mar, Brasília, DF, Brazil; Grant No. 84/2010) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq—Instituto Nacional de Ciência e Tecnologia de Toxicologia Aquática, Brasília, DF, Brazil; Grant No. 573949/2008-5) are acknowledged for their financial support. The Coral Vivo Project and its sponsors Petrobras, through the Petrobras Environmental Program, and Arraial d’Ajuda Eco Parque are acknowledged for their support in field research. A. Bianchini (Proc. # 304430/2009-9) and C.B. Castro (Proc. # 303970/2010-3) are research fellows from CNPq. A. Bianchini is supported by the International Canada Research Chair Program (IDRC). D.C. Luz was a graduate fellow from CAPES.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Topic Editor Dr. Anastazia Banaszak
Rights and permissions
About this article
Cite this article
Luz, D.C., Zebral, Y.D., Klein, R.D. et al. Oxidative stress in the hydrocoral Millepora alcicornis exposed to CO2-driven seawater acidification. Coral Reefs 37, 571–579 (2018). https://doi.org/10.1007/s00338-018-1681-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-018-1681-2