Abstract
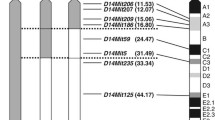
Streptozotocin (STZ) has been widely used to induce diabetes in rodents. Strain-dependent variation in susceptibility to STZ has been reported; however, the gene(s) responsible for STZ susceptibility has not been identified. Here, we utilized the A/J-11SM consomic strain and a set of chromosome 11 (Chr. 11) congenic strains developed from A/J-11SM to identify a candidate STZ-induced diabetes susceptibility gene. The A/J strain exhibited significantly higher susceptibility to STZ-induced diabetes than the A/J-11SM strain, confirming the existence of a susceptibility locus on Chr. 11. We named this locus Stzds1 (STZ-induced diabetes susceptibility 1). Congenic mapping using the Chr. 11 congenic strains indicated that the Stzds1 locus was located between D11Mit163 (27.72 Mb) and D11Mit51 (36.39 Mb). The Mpg gene, which encodes N-methylpurine DNA glycosylase (MPG), a ubiquitous DNA repair enzyme responsible for the removal of alkylated base lesions in DNA, is located within the Stzds1 region. There is a close relationship between DNA alkylation at an early stage of STZ action and the function of MPG. A Sanger sequence analysis of the Mpg gene revealed five polymorphic sites in the A/J genome. One variant, p.Ala132Ser, was located in a highly conserved region among rodent species and in the minimal region for retained enzyme activity of MPG. It is likely that structural alteration of MPG caused by the p.Ala132Ser mutation elicits increased recognition and excision of alkylated base lesions in DNA by STZ.



Similar content being viewed by others
References
Babaya N, Ikegami H, Fujisawa T, Nojima K, Itoi-Babaya M, Inoue K, Ohno T, Shibata M, Ogihara T (2005) Susceptibility to streptozotocin-induced diabetes is mapped to mouse chromosome 11. Biochem Biophys Res Commun 328:158–164
Bhatnagar S, Oler AT, Rabaglia ME, Stapleton DS, Schueler KL, Truchan NA, Worzella SL, Stoehr JP, Clee SM, Yandell BS, Keller MP, Thurmond DC, Attie AD (2011) Positional cloning of a type 2 diabetes quantitative trait locus; tomosyn-2, a negative regulator of insulin secretion. PLoS Genet 7:e1002323
Burkart V, Wang ZQ, Radons J, Heller B, Herceg Z, Stingl L, Wagner EF, Kolb H (1999) Mice lacking the poly(ADP-ribose) polymerase gene are resistant to pancreatic beta-cell destruction and diabetes development induced by streptozocin. Nat Med 5:314–319
Burns N, Gold B (2007) The effect of 3-methyladenine DNA glycosylase-mediated DNA repair on the induction of toxicity and diabetes by the beta-cell toxicant streptozotocin. Toxicol Sci 95:391–400
Cardinal JW, Margison GP, Mynett KJ, Yates AP, Cameron DP, Elder RH (2001) Increased susceptibility to streptozotocin-induced beta-cell apoptosis and delayed autoimmune diabetes in alkylpurine-DNA-N-glycosylase-deficient mice. Mol Cell Biol 21:5605–5613
Choi Y, Chan AP (2015) PROVEAN web server: a tool to predict the functional effect of amino acid substitutions and indels. Bioinformatics 31:2745–2477
Clee SM, Yandell BS, Schueler KM, Rabaglia ME, Richards OC, Raines SM, Kabara EA, Klass DM, Mui ET, Stapleton DS, Gray-Keller MP, Young MB, Stoehr JP, Lan H, Boronenkov I, Raess PW, Flowers MT, Attie AD (2006) Positional cloning of Sorcs1, a type 2 diabetes quantitative trait locus. Nat Genet 38:688–693
Daimon M, Oizumi T, Toriyama S, Karasawa S, Jimbu Y, Wada K, Kameda W, Susa S, Muramatsu M, Kubota I, Kawata S, Kato T (2009) Association of the Ser326Cys polymorphism in the OGG1 gene with type 2 DM. Biochem Biophys Res Commun 386:26–29
Dooley J, Tian L, Schonefeldt S, Delghingaro-Augusto V, Garcia-Perez JE, Pasciuto E, Di Marino D, Carr EJ, Oskolkov N, Lyssenko V, Franckaert D, Lagou V, Overbergh L, Vandenbussche J, Allemeersch J, Chabot-Roy G, Dahlstrom JE, Laybutt DR, Petrovsky N, Socha L, Gevaert K, Jetten AM, Lambrechts D, Linterman MA, Goodnow CC, Nolan CJ, Lesage S, Schlenner SM, Liston A (2016) Genetic predisposition for beta cell fragility underlies type 1 and type 2 diabetes. Nat Genet 48:519–527
Festing MF (1996) Origins and characteristics of inbred strains of mice. In: Lyon MF, Rasten S, Brown SDM (eds) Genetic variants and strains of the laboratory mouse. Oxford University Press, New York, pp 1537–1576
Gonzalez C, Cuvellier S, Hue-Beauvais C, Lévi-Strauss M (2003) Genetic control of non obese diabetic mice susceptibility to high-dose streptozotocin-induced diabetes. Diabetologia 46:1291–1295
Hada N, Kobayashi M, Fujiyoshi M, Ishikawa A, Kuga M, Nishimura M, Ebihara S, Ohno T, Horio F (2008) Quantitative trait loci for impaired glucose tolerance in nondiabetic SM/J and A/J mice. Physiol Genomics 35:65–74
Hadjivassiliou V, Green MH, James RF, Swift SM, Clayton HA, Green IC (1998) Insulin secretion, DNA damage, and apoptosis in human and rat islets of Langerhans following exposure to nitric oxide, peroxynitrite, and cytokines. Nitric Oxide 2:429–441
Kaku K, Fiedorek FT Jr, Province M, Permutt MA (1988) Genetic analysis of glucose tolerance in inbred mouse strains: evidence for polygenic control. Diabetes 37:707–713
Kaku K, McGill J, Province M, Permutt MA (1989) A single major gene controls most of the difference in susceptibility to streptozotocin-induced diabetes between C57BL/6J and C3H/HeJ mice. Diabetologia 32:716–723
Kikutani H, Makino S (1992) The murine autoimmune diabetes model: NOD and related strains. Adv Immunol 51:285–322
Kobayashi M, Ohno T, Hada N, Fujiyoshi M, Kuga M, Nishimura M, Murai A, Horio F (2010) Genetic analysis of abdominal fat distribution in SM/J and A/J mice. J Lipid Res 51:3463–3469
Kolb H (1987) Mouse models of insulin dependent diabetes: low-dose streptozocin-induced diabetes and nonobese diabetic (NOD) mice. Diabetes Metab Rev 3:751–778
Lenzen S (2008) The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia 51:216–226
Liston A, Todd JA, Lagou V (2017) Beta-cell fragility as a common underlying risk factor in type 1 and type 2 diabetes. Trends Mol Med 23:181–194
Masutani M, Suzuki H, Kamada N, Watanabe M, Ueda O, Nozaki T, Jishage K, Watanabe T, Sugimoto T, Nakagama H, Ochiya T, Sugimura T (1999) Poly(ADP-ribose) polymerase gene disruption conferred mice resistant to streptozotocin-induced diabetes. Proc Natl Acad Sci USA 96:2301–2304
Nadeau JH, Singer JB, Matin A, Lander ES (2000) Analysing complex genetic traits with chromosome substitution strains. Nat Genet 24:221–225
Nishimura M, Hirayama N, Serikawa T, Kanehira K, Matsushima Y, Katoh H, Wakana S, Kojima A, Hiai H (1995) The SMXA: a new set of recombinant inbred strain of mice consisting of 26 substrains and their genetic profile. Mamm Genome 6:850–857
Ohno T, Hata K, Baba T, Io F, Kobayashi M, Horio F, Nishimura M (2012) Establishment of consomic strains derived from A/J and SM/J mice for genetic analysis of complex traits. Mamm Genome 23:764–769
Pataer A, Nishimura M, Kamoto T, Ichioka K, Sato M, Hiai H (1997) Genetic resistance to urethan-induced pulmonary adenomas in SMXA recombinant inbred mouse strains. Cancer Res 57:2904–2908
Pieper AA, Brat DJ, Krug DK, Watkins CC, Gupta A, Blackshaw S, Verma A, Wang ZQ, Snyder SH (1999) Poly(ADP-ribose) polymerase-deficient mice are protected from streptozotocin-induced diabetes. Proc Natl Acad Sci USA 96:3059–3064
Rogner UC, Avner P (2003) Congenic mice: cutting tools for complex immune disorders. Nat Rev Immunol 3:243–252
Rossini AA, Appel MC, Williams RM, Like AA (1977) Genetic influence of the streptozotocin-induced insulitis and hyperglycemia. Diabetes 26:916–920
Roy R, Kumar A, Lee JC, Mitra S (1996) The domains of mammalian base excision repair enzyme N-methylpurine-DNA glycosylase. Interaction, conformational change, and role in DNA binding and damage recognition. J Biol Chem 271:23690–23697
Roy R, Biswas T, Hazra TK, Roy G, Grabowski DT, Izumi T, Srinivasan G, Mitra S (1998) Specific interaction of wild-type and truncated mouse N-methylpurine-DNA glycosylase with ethenoadenine-containing DNA. Biochemistry 37:580–589
Singer JB, Hill AE, Burrage LC, Olszens KR, Song J, Justice M, O’Brien WE, Conti DV, Witte JS, Lander ES, Nadeau JH (2004) Genetic dissection of complex traits with chromosome substitution strains of mice. Science 304:445–448
Stylianou IM, Clinton M, Keightley PD, Pritchard C, Tymowska-Lalanne Z, Bunger L, Horvat S (2005) Microarray gene expression analysis of the Fob3b obesity QTL identifies positional candidate gene Sqle and perturbed cholesterol and glycolysis pathways. Physiol Genomics 20:224–232
Szkudelski T (2012) Streptozotocin-nicotinamide-induced diabetes in the rat. Characteristics of the experimental model. Exp Biol Med 237:481–490
Takada T, Mita A, Maeno A, Sakai T, Shitara H, Kikkawa Y, Moriwaki K, Yonekawa H, Shiroishi T (2008) Mouse inter-subspecific consomic strains for genetic dissection of quantitative complex traits. Genome Res 18:500–508
Tanaka S, Mizorogi T, Nishijima K, Kuwahara S, Tsujio M, Aoyama H, Taguchi C, Kobayashi M, Horio F, Ohno T (2009) Body and major organ weights of A/J-Chr11SM consomic mice. Exp Anim 58:357–361
Thameem F, Puppala S, Lehman DM, Stern MP, Blangero J, Abboud HE, Duggirala R, Habib SL (2009) The Ser(326)Cys polymorphism of 8-oxoguanine glycosylase 1 (OGG1) is associated with type 2 diabetes in Mexican Americans. Hum Hered 70:97–101
Ueda H, Ikegami H, Yamato E, Fu J, Fukuda M, Shen G, Kawaguchi Y, Takekawa K, Fujioka Y, Fujisawa T, Nakagawa Y, Hamada Y, Shibata M, Ogihara T (1995) The NSY mouse: a new animal model of spontaneous NIDDM with moderate obesity. Diabetologia 38:503–508
Wyatt MD, Allan JM, Lau AY, Ellenberger TE, Samson LD (1999) 3-Methyladenine DNA glycosylases: structure, function, and biological importance. Bioessays 21:668–676
Yamamoto H, Uchigata Y, Okamoto H (1981) Streptozotocin and alloxan induce DNA strand breaks and poly(ADP-ribose)synthetase in pancreatic islets. Nature 294:284–286
Acknowledgements
This work was supported by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (23500494 to T. Ohno).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Maegawa, T., Miyasaka, Y., Kobayashi, M. et al. Congenic mapping and candidate gene analysis for streptozotocin-induced diabetes susceptibility locus on mouse chromosome 11. Mamm Genome 29, 273–280 (2018). https://doi.org/10.1007/s00335-018-9742-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00335-018-9742-y