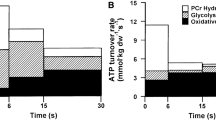
Abstract
Repeated bouts of physical exercise, i.e., training, induce mitochondrial biogenesis and result in improved physical performance and attenuation of glycogen breakdown during submaximal exercise. It has been suggested that as a consequence of the increased mitochondrial volume, a smaller degree of metabolic stress (e.g., smaller increases in ADP and Pi) is required to maintain mitochondrial respiration in the trained state during exercise at the same absolute intensity. The lower degree of Pi accumulation is believed to account for the diminished glycogen breakdown, since Pi is a substrate for glycogen phosphorylase, the rate-limiting enzyme for glycogenolysis. However, in this review, we present an alternative explanation for the diminished glycogen breakdown. Thus, the lower degree of metabolic stress after training is also associated with smaller increases in AMP (free concentration during contraction at specific intracellular sites) and this results in less activation of phosphorylase b (the non-phosphorylated form of phosphorylase), resulting in diminished glycogen breakdown. Concomitantly, the smaller accumulation of Pi, which interferes with cross-bridge function and intracellular Ca2+ handling, contributes to the increased fatigue resistance. The delay in glycogen depletion also contributes to enhanced performance during prolonged exercise by functioning as an energy reserve.


Similar content being viewed by others
References
Ahlborg B, Bergström J, Ekelund LG, Guarnieri G, Harris RC, Hultman E, Nordesjö LO (1972) Muscle metabolism during isometric exercise performed at constant force. J Appl Physiol 33:224–228
Allen DG, Lamb GD, Westerblad H (2008) Skeletal muscle fatigue: cellular mechanisms. Physiol Rev 88:287–332
Bergström J, Hermansen L, Hultman E, Saltin B (1967) Diet, muscle glycogen and physical performance. Acta Physiol Scand 71:140–150
Broberg S, Katz A, Sahlin K (1988) Propranolol enhances adenine nucleotide degradation in human muscle during exercise. J Appl Physiol 65:2478–2483
Brostrom CO, Hunkeler FL, Krebs EG (1971) The relation of skeletal muscle phosphorylase kinase by Ca2+. J Biol Chem 246:1961–1967
Brown DH, Cori CF (1961) Animal and plant polysaccharide phosphorylase. Enzymes 5:207–228
Cairns SP, Westerblad H, Allen DG (1993) Changes of tension and [Ca2+]i during beta-adrenoceptor activation of single, intact fibres from mouse skeletal muscle. Pflugers Arch 425:150–155
Chasiotis D (1983) The regulation of glycogen phosphorylase and glycogen breakdown in human skeletal muscle. Acta Physiol Scand Suppl 518:1–68
Chasiotis D (1985) Effects of adrenaline infusion on cAMP and glycogen phosphorylase in fast-twitch and slow-twitch rat muscles. Acta Physiol Scand 125:537–540
Chasiotis D, Hultman E (1983) The effect of circulatory occlusion on the glycogen phosphorylase- synthetase system in human skeletal muscle. J Physiol 345:167–173
Chasiotis D, Hultman E (1985) The effect of adrenaline infusion on the regulation of glycogenolysis in human muscle during isometric contraction. Acta Physiol Scand 123:55–60
Chasiotis D, Sahlin K, Hultman E (1982) Regulation of glycogenolysis in human muscle at rest and during exercise. J Appl Physiol 53:708–715
Chasiotis D, Sahlin K, Hultman E (1983) Regulation of glycogenolysis in human muscle in response to epinephrine infusion. J Appl Physiol 54:45–50
Chasiotis D, Edström L, Sahlin K, Sjohölm H (1985) Activation of glycogen phosphorylase by electrical stimulation of isolated fast-twitch and slow-twitch muscles from rat. Acta Physiol Scand 123:43–47
Chesley A, Heigenhauser GJ, Spriet LL (1996) Regulation of muscle glycogen phosphorylase activity following short- term endurance training. Am J Physiol 270:E328–E335
Chin ER, Allen DG (1997) Effects of reduced muscle glycogen concentration on force, Ca2+ release and contractile protein function in intact mouse skeletal muscle. J Physiol Lond 498:17–29
Coggan AR, Kohrt WM, Spina RJ, Bier DM, Holloszy JO (1990) Endurance training decreases plasma glucose turnover and oxidation during moderate-intensity exercise in men. J Appl Physiol 68:990–996
Conlee RK, McLane JA, Rennie MJ, Winder WW, Holloszy JO (1979) Reversal of phosphorylase activation in muscle despite continued contractile activity. Am J Physiol 237:R291–R296
Constable SH, Favier RJ, Holloszy JO (1986) Exercise and glycogen depletion: effects on ability to activate muscle phosphorylase. J Appl Physiol 60:1518–1523
Constable SH, Favier RJ, McLane JA, Fell RD, Chen M, Holloszy JO (1987) Energy metabolism in contracting rat skeletal muscle: adaptation to exercise training. Am J Physiol 253:C316–C322
Dahlstedt AJ, Katz A, Wieringa B, Westerblad H (2000) Is creatine kinase responsible for fatigue? Studies of isolated skeletal muscle deficient in creatine kinase. FASEB J 14:982–990
Danforth WH, Helmreich E (1964) Regulation of glycolysis in muscle. I. The conversion of phosphorylase b to a in frog sartorius muscle. J Biol Chem 239:3133–3138
Danforth WH, Lyon JB Jr (1964) Glycogenolysis during tetanic contraction of isolated mouse muscles in the presence and absence of phosphorylase a. J Biol Chem 239:1050–4047
Danforth WH, Helmreich E, Cori CF (1962) The effect of contraction and of epinephrine on the phosphorylase activity of frog sartorius muscle. Proc Natl Acad Sci USA 48:1191–1199
Dudley GA, Terjung RL (1985) Influence of aerobic metabolism on IMP accumulation in fast-twitch muscle. Am J Physiol 248:C37–C42
Dudley GA, Tullson PC, Terjung RL (1987) Influence of mitochondrial content on the sensitivity of respiratory control. J Biol Chem 262:9109–9114
Ekblom B (1968) Effect of physical training on oxygen transport system in man. Acta Physiol Scand Suppl 328:1–45
Favier RJ, Constable SH, Chen M, Holloszy JO (1986) Endurance exercise training reduces lactate production. J Appl Physiol 61:885–889
Fischer EH (2013) Cellular regulation by protein phosphorylation. Biochem Biophys Res Commun 430:865–867
Frank P, Katz A, Andersson E, Sahlin K (2013) Acute exercise reverses starvation-mediated insulin resistance in humans. Am J Physiol Endocrinol Metab 304:E436–E443
Galbo H, Holst JJ, Christensen NJ (1979) The effect of different diets and of insulin on the hormonal response to prolonged exercise. Acta Physiol Scand 107:19–32
Gollnick PD, Saltin B (1982) Significance of skeletal muscle oxidative enzyme enhancement with endurance training. Clin Physiol 2:1–12
Green HJ, Helyar R, Ball-Burnett M, Kowalchuk N, Symon S, Farrance B (1992) Metabolic adaptations to training precede changes in muscle mitochondrial capacity. J Appl Physiol 72:484–491
Green HJ, Bombardier E, Burnett ME, Smith IC, Tupling SM, Ranney DA (2009) Time-dependent effects of short-term training on muscle metabolism during the early phase of exercise. Am J Physiol Regul Integr Comp Physiol 297:R1383–R1391
Harris RC, Hultman E, Nordesjö LO (1974) Glycogen, glycolytic intermediates and high-energy phosphates determined in biopsy samples of musculus quadriceps femoris of man at rest. Methods and variance of values. Scand J Clin Lab Invest 33:109–120
Harris RC, Hultman E, Kaijser L, Nordesjö LO (1975) The effect of circulatory occlusion on isometric exercise capacity and energy metabolism of the quadriceps muscle in man. Scand J Clin Lab Invest 35:87–95
Helander I, Westerblad H, Katz A (2002) Effects of glucose on contractile function, [Ca2+]i, and glycogen in isolated mouse skeletal muscle. Am J Physiol Cell Physiol 282:C1306–C1312
Hespel P, Richter EA (1992) Mechanism linking glycogen concentration and glycogenolytic rate in perfused contracting rat skeletal muscle. Biochem J 284:777–780
Holloszy JO (1967) Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J Biol Chem 242:2278–2282
Holloszy JO (2011) Regulation of mitochondrial biogenesis and GLUT4 expression by exercise. Compr Physiol 1:921–940
Hultman E, Sjöholm H (1983a) Energy metabolism and contraction force of human skeletal muscle in situ during electrical stimulation. J Physiol 345:525–532
Hultman E, Sjöholm H (1983b) Substrate availability. In: Knuttgen HG, Vogel JA, Poortmans JR (eds) International Symposium on Biochemistry of Exercise. Human Kinetics, Boston, pp 63–75
Hunter RW, Treebak JT, Wojtaszewski JF, Sakamoto K (2011) Molecular mechanism by which AMP-activated protein kinase activation promotes glycogen accumulation in muscle. Diabetes 60:766–774
Jiao Y, Shashkina E, Shashkin P, Hansson A, Katz A (1999) Manganese sulfate-dependent glycosylation of endogenous glycoproteins in human skeletal muscle is catalyzed by a nonglucose 6-P-dependent glycogen synthase and not glycogenin. Biochim Biophys Acta 1427:1–12
Johnson LN (1992) Glycogen phosphorylase: control by phosphorylation and allosteric effectors. FASEB J 6:2274–2282
Kabbara AA, Nguyen LT, Stephenson GM, Allen DG (2000) Intracellular calcium during fatigue of cane toad skeletal muscle in the absence of glucose. J Muscle Res Cell Motil 21:481–489
Karlsson J, Nordesjö LO, Jorfeldt L, Saltin B (1972) Muscle lactate, ATP, and CP levels during exercise after physical training in man. J Appl Physiol 33:199–203
Kasvinsky PJ, Meyer WL (1977) The effect of pH and temperature on the kinetics of native and altered glycogen phosphorylase. Arch Biochem Biophys 181:616–631
Katz A (1988) G-1,6-P2, glycolysis, and energy metabolism during circulatory occlusion in human skeletal muscle. Am J Physiol 255:C140–C144
Katz A (1997) Differential responses of glycogen synthase to ischaemia and ischaemic contraction in human skeletal muscle. Exp Physiol 82:203–211
Katz A, Sahlin K (1990) Role of oxygen in regulation of glycolysis and lactate production in human skeletal muscle. Exercise Sport Sci Rev 18:1–28
Katz A, Sahlin K, Henriksson J (1986) Muscle ammonia metabolism during isometric contraction in humans. Am J Physiol 250:C834–C840
Katz A, Andersson DC, Yu J, Norman B, Sandström ME, Wieringa B, Westerblad H (2003) Contraction-mediated glycogenolysis in mouse skeletal muscle lacking creatine kinase: the role of phosphorylase b activation. J Physiol 553:523–531
Kiens B, Essen-Gustavsson B, Christensen NJ, Saltin B (1993) Skeletal muscle substrate utilization during submaximal exercise in man: effect of endurance training. J Physiol 469:459–478
Larsson J, Hultman E (1979) The effect of long-term arterial occlusion on energy metabolism of the human quadriceps muscle. Scand J Clin Lab Invest 39:257–264
Lee SH, Davis EJ (1979) Carboxylation and decarboxylation reactions. Anaplerotic flux and removal of citrate cycle intermediates in skeletal muscle. J Biol Chem 254:420–430
Lowry OH, Passonneau JV, Hasselberger FX, Schulz DW (1964a) Effect of ischemia on known substrates and cofactors of the glycolytic pathway in brain. J Biol Chem 239:18–30
Lowry OH, Schulz DW, Passonneau JV (1964b) Effects of adenylic acid on the kinetics of phosphorylase a. J Biol Chem 239:1947–1953
Meyer RA, Terjung RL (1979) Differences in ammonia and adenylate metabolism in contracting fast and slow muscle. Am J Physiol 237:C111–C118
Meyer RA, Terjung RL (1980) AMP deamination and IMP reamination in working skeletal muscle. Am J Physiol 239:C32–C38
Miyamoto L, Toyoda T, Hayashi T, Yonemitsu S, Nakano M, Tanaka S, Ebihara K, Masuzaki H, Hosoda K, Ogawa Y, Inoue G, Fushiki T, Nakao K (2007) Effect of acute activation of 5′-AMP-activated protein kinase on glycogen regulation in isolated rat skeletal muscle. J Appl Physiol 102:1007–1013
Morgan HE, Parmeggiani A (1964a) Regulation of glycogenolysis in muscle. II. Control of glycogen phosphorylase reaction in isolated perfused heart. J Biol Chem 239:2435–2439
Morgan HE, Parmeggiani A (1964b) Regulation of glycogenolysis in muscle. III. control of muscle glycogen phosphorylase activity. J Biol Chem 239:2440–2445
Newsholme EA, Leech AR (1985) Biochemistry for the medical sciences. Wiley, Chichester
Nielsen J, Schroder HD, Rix CG, Ørtenblad N (2009) Distinct effects of subcellular glycogen localization on tetanic relaxation time and endurance in mechanically skinned rat skeletal muscle fibres. J Physiol 587:3679–3690
Nielsen J, Holmberg HC, Schroder HD, Saltin B, Ørtenblad N (2011) Human skeletal muscle glycogen utilization in exhaustive exercise: role of subcellular localization and fibre type. J Physiol 589:2871–2885
Ørtenblad N, Westerblad H, Nielsen J (2013) Muscle glycogen stores and fatigue. J Physiol 591:4405–4413
Phillips SM, Green HJ, Tarnopolsky MA, Heigenhauser GJ, Grant SM (1996) Progressive effect of endurance training on metabolic adaptations in working skeletal muscle. Am J Physiol 270:E265–E272
Piras R, Staneloni R (1969) In vivo regulation of rat muscle glycogen synthetase activity. Biochemistry 8:2153–2160
Posner JB, Stern R, Krebs EG (1965) Effects of electrical stimulation and epinephrine on muscle phosphorylase, phosphorylase b kinase, and adenosine 3′,5′-phosphates. J Biol Chem 240:982–985
Preisler N, Orngreen MC, Echaniz-Laguna A, Laforet P, Lonsdorfer-Wolf E, Doutreleau S, Geny B, Akman HO, DiMauro S, Vissing J (2012) Muscle phosphorylase kinase deficiency: a neutral metabolic variant or a disease? Neurology 78:265–268
Rahim ZH, Perrett D, Lutaya G, Griffiths JR (1980) Metabolic adaptation in phosphorylase kinase deficiency. Changes in metabolite concentrations during tetanic stimulation of mouse leg muscles. Biochem J 186:331–341
Ren JM, Hultman E (1989) Regulation of glycogenolysis in human skeletal muscle. J Appl Physiol 67:2243–2248
Ren JM, Hultman E (1990) Regulation of phosphorylase a activity in human skeletal muscle. J Appl Physiol 69:919–923
Ren JM, Broberg S, Sahlin K, Hultman E (1990) Influence of reduced glycogen level on glycogenolysis during short-term stimulation in man. Acta Physiol Scand 139:467–474
Ren JM, Gulve EA, Cartee GD, Holloszy JO (1992) Hypoxia causes glycogenolysis without an increase in percent phosphorylase a in rat skeletal muscle. Am J Physiol 263:E1086–E1091
Richter EA, Galbo H (1986) High glycogen levels enhance glycogen breakdown in isolated contracting skeletal muscle. J Appl Physiol 61:827–831
Richter EA, Sonne B, Christensen NJ, Galbo H (1981) Role of epinephrine for muscular glycogenolysis and pancreatic hormonal secretion in running rats. Am J Physiol 240:E526–E532
Richter EA, Ruderman NB, Gavras H, Belur ER, Galbo H (1982) Muscle glycogenolysis during exercise: dual control by epinephrine and contractions. Am J Physiol 242:E25–E32
Roach PJ (2002) Glycogen and its metabolism. Curr Mol Med 2:101–120
Rush JW, Spriet LL (2001) Skeletal muscle glycogen phosphorylase a kinetics: effects of adenine nucleotides and caffeine. J Appl Physiol 91:2071–2078
Sahlin K, Harris RC (2011) The creatine kinase reaction: a simple reaction with functional complexity. Amino Acids 40:1363–1367
Sahlin K, Broberg S, Katz A (1989) Glucose formation in human skeletal muscle. Influence of glycogen content. Biochem J 258:911–913
Sahlin K, Katz A, Broberg S (1990) Tricarboxylic acid cycle intermediates in human muscle during prolonged exercise. Am J Physiol 259: C834-C841 [published errata appear in Am J Physiol 1995 Feb; 268(2 Pt 1):section C following table of contents and 1995 Jun; 268(6 Pt 3):section C following table of contents]
Sahlin K, Tonkonogi M, Söderlund K (1998) Energy supply and muscle fatigue in humans. Acta Physiol Scand 162:261–266
Saltin B, Blomqvist G, Mitchell JH, Johnson RL, Jr, Wildenthal K, Chapman CB (1968) Response to exercise after bed rest and after training. Circulation 38:VII1–VII78
Spencer MK, Katz A (1991) Role of glycogen in control of glycolysis and IMP formation in human muscle during exercise. Am J Physiol 260:E859–E864
Spencer MK, Yan Z, Katz A (1992) Effect of low glycogen on carbohydrate and energy metabolism in human muscle during exercise. Am J Physiol 262:C975–C979
Spriet LL, Berardinucci L, Marsh DR, Campbell CB, Graham TE (1990) Glycogen content has no effect on skeletal muscle glycogenolysis during short-term tetanic stimulation. J Appl Physiol 68:1883–1888
Stalmans W, Gevers G (1981) The catalytic activity of phosphorylase b in the liver. With a note on the assay in the glycogenolytic direction. Biochem J 200:327–336
van Deursen J, Heerschap A, Oerlemans F, Ruitenbeek W, Jap P, ter Laak H, Wieringa B (1993) Skeletal muscles of mice deficient in muscle creatine kinase lack burst activity. Cell 74:621–631
van Deursen J, Ruitenbeek W, Heerschap A, Jap P, ter Laak H, Wieringa B (1994) Creatine kinase (CK) in skeletal muscle energy metabolism: a study of mouse mutants with graded reduction in muscle CK expression. Proc Natl Acad Sci U S A 91:9091–9095
Westerblad H, Dahlstedt AJ, Lännergren J (1998) Mechanisms underlying reduced maximum shortening velocity during fatigue of intact, single fibres of mouse muscle. J Physiol 510:269–277
Young ME, Radda GK, Leighton B (1996) Activation of glycogen phosphorylase and glycogenolysis in rat skeletal muscle by AICAR–an activator of AMP-activated protein kinase. FEBS Lett 382:43–47
Acknowledgments
The authors’ research is supported by grants from the Swedish National Center for Sports Research and the Swedish Research Council (to H.W.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Katz, A., Westerblad, H. Regulation of glycogen breakdown and its consequences for skeletal muscle function after training. Mamm Genome 25, 464–472 (2014). https://doi.org/10.1007/s00335-014-9519-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00335-014-9519-x