Abstract
Purpose
This study was conducted in order to investigate the topological organization of functional and structural brain networks in diabetic kidney disease (DKD) and its potential clinical relevance.
Methods
Two hundred two subjects (62 DKD patients, 60 diabetes mellitus [DM] patients, and 80 healthy controls) underwent laboratory examination, neuropsychological test, and magnetic resonance imaging (MRI). Large-scale functional and structural brain networks were constructed and graph theoretical network analyses were performed. The effect of renal function on brain functional and structural networks in DKD patients was further evaluated. Correlations were performed between network properties and neuropsychological scores and clinical variables.
Results
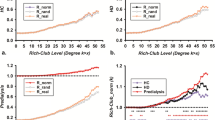
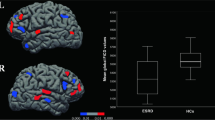
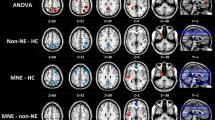
Progressing deteriorated global and local network topology organizations (especially for functional network) were observed for DKD patients compared with control subjects (all p < 0.05, Bonferroni-corrected), with intermediate values for the patients with DM. DKD patients showed normally appearing functional–structural coupling compared with controls, while DM patients manifested functional–structural decoupling (p < 0.05, Bonferroni-corrected). Impaired kidney function markedly affected functional and structural network organization in DKD patients (all p < 0.05). Urea nitrogen correlated with global and local efficiency in the structural networks (r = − 0.551, p < 0.001; r = − 0.476, p < 0.001, respectively). Global and local efficiency in the structural networks and normalized characteristic path length in the functional networks were associated with information processing speed and/or psychomotor speed.
Conclusion
DKD patients showed enhanced functional and structural brain network disruption and normally appearing functional–structural coupling compared with DM patients, which correlated with kidney function, renal toxins, and cognitive performance.
Key Points
• DKD patients showed markedly disrupted functional and structural brain network efficiency measures compared with DM patients and healthy controls.
• Reduced kidney function clearly deteriorated functional and structural brain networks in DKD patients.
• DKD patients displayed normally appearing functional–structural coupling compared with DM patients.






Similar content being viewed by others
Abbreviations
- γ :
-
Normalized clustering coefficient
- λ :
-
Normalized characteristic path length
- σ :
-
Small-worldness
- AAL:
-
Automated anatomical labeling
- ADA:
-
American Diabetes Association
- ANCOVA:
-
Analysis of covariance
- BUN:
-
Blood urea nitrogen
- Cp:
-
Clustering coefficient
- Cyst C:
-
Cystatin C
- DKD:
-
Diabetic kidney disease
- DM:
-
Diabetes mellitus
- DPARSF:
-
Data Processing Assistant for Resting-State fMRI
- DS:
-
Deep subcortical
- DTI:
-
Diffusion tensor imaging
- Eg:
-
Global efficiency
- eGFR:
-
Estimated glomerular filtration rate
- Eloc:
-
Local efficiency
- FBG:
-
Fasting blood glucose
- HbA1c:
-
Hemoglobin A1c
- HDL-C:
-
High-density lipoprotein cholesterol
- LDL-C:
-
Low-density lipoprotein cholesterol
- Lp:
-
Characteristic path length
- LTT:
-
Line Tracing Test
- MMSE:
-
Mini-Mental State Examination
- MoCA:
-
Montreal Cognitive Assessment
- MRI:
-
Magnetic resonance imaging
- NCT-A:
-
Number Connection Test type-A
- PV:
-
Periventricular
- rs-fMRI:
-
Resting-state functional magnetic resonance imaging
- SAS:
-
Self-Rating Anxiety Scale
- Scr:
-
Serum creatinine
- SDS:
-
Self-Rating Depression Scale
- SDT:
-
Serial Dotting Test
- TC:
-
Total cholesterol
- TIV:
-
Total intracranial volume
- Tri:
-
Triglycerides
- UA:
-
Uric acid
- WML:
-
White matter lesions
References
Ingelfinger JR, Jarcho JA (2017) Increase in the incidence of diabetes and its implications. N Engl J Med 376:1473–1474
Polonsky KS (2012) The past 200 years in diabetes. N Engl J Med 367:1332–1340
Yang W, Lu J, Weng J et al (2010) Prevalence of diabetes among men and women in China. N Engl J Med 362:1090–1101
Gregg EW, Li Y, Wang J et al (2014) Changes in diabetes-related complications in the United States, 1990-2010. N Engl J Med 370:1514–1523
[No authors listed] (2015) Diabetic kidney disease: what does the next era hold? Lancet Diabetes Endocrinol 3:665
de Boer IH (2017) A new chapter for diabetic kidney disease. N Engl J Med 377:885–887
Reijmer YD, Leemans A, Brundel M, Kappelle LJ, Biessels GJ, Utrecht Vascular Cognitive Impairment Study Group (2013) Disruption of the cerebral white matter network is related to slowing of information processing speed in patients with type 2 diabetes. Diabetes 62:2112–2115
van Bussel FC, Backes WH, van Veenendaal TM et al (2016) Functional brain networks are altered in type 2 diabetes and prediabetes: signs for compensation of cognitive decrements? The Maastricht study. Diabetes 65:2404–2413
Sink KM, Divers J, Whitlow CT et al (2015) Cerebral structural changes in diabetic kidney disease: African American-Diabetes Heart Study MIND. Diabetes Care 38:206–212
Murea M, Hsu FC, Cox AJ et al (2015) Structural and functional assessment of the brain in European Americans with mild-to-moderate kidney disease: Diabetes Heart Study-MIND. Nephrol Dial Transplant 30:1322–1329
Bressler SL, Menon V (2010) Large-scale brain networks in cognition: emerging methods and principles. Trends Cogn Sci 14:277–290
Bullmore E, Sporns O (2009) Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci 10:186–198
Bullmore E, Sporns O (2012) The economy of brain network organization. Nat Rev Neurosci 13:336–349
Vaessen MJ, Jansen JF, Vlooswijk MC et al (2012) White matter network abnormalities are associated with cognitive decline in chronic epilepsy. Cereb Cortex 22:2139–2147
Bassett DS, Bullmore ET (2009) Human brain networks in health and disease. Curr Opin Neurol 22:340–347
Zheng G, Wen J, Zhang L et al (2014) Altered brain functional connectivity in hemodialysis patients with end-stage renal disease: a resting-state functional MR imaging study. Metab Brain Dis 29:777–786
Zhang LJ, Zheng G, Zhang L et al (2012) Altered brain functional connectivity in patients with cirrhosis and minimal hepatic encephalopathy: a functional MR imaging study. Radiology 265:528–536
Zhang LJ, Zheng G, Zhang L et al (2014) Disrupted small world networks in patients without overt hepatic encephalopathy: a resting state fMRI study. Eur J Radiol 83:1890–1899
American Diabetes Association (2018) 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2018. Diabetes Care 41:S13–S27
KDOQI (2007) KDOQI clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease. Am J Kidney Dis 49:S12–S154
Van der Elst W, Van Boxtel MP, Van Breukelen GJ, Jolles J (2006) The Stroop color-word test: influence of age, sex, and education; and normative data for a large sample across the adult age range. Assessment 13:62–79
Zung WW (1971) A rating instrument for anxiety disorders. Psychosomatics 12:371–379
Faravelli C, Albanesi G, Poli E (1986) Assessment of depression: a comparison of rating scales. J Affect Disord 11:245–253
Wang YF, Kong X, Lu GM, Zhang LJ (2017) Diabetes mellitus is associated with more severe brain spontaneous activity impairment and gray matter loss in patients with cirrhosis. Sci Rep 7:7775
Zhang LJ, Wen J, Liang X et al (2016) Brain default mode network changes after renal transplantation: a diffusion-tensor imaging and resting-state functional MR imaging study. Radiology 278:485–495
Chao-Gan Y, Yu-Feng Z (2010) DPARSF: a MATLAB toolbox for “pipeline” data analysis of resting-state fMRI. Front Syst Neurosci 4:13
Cui Z, Zhong S, Xu P, He Y, Gong G (2013) PANDA: a pipeline toolbox for analyzing brain diffusion images. Front Hum Neurosci 7:42
Tzourio-Mazoyer N, Landeau B, Papathanassiou D et al (2002) Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15:273–289
Wang J, Wang X, Xia M, Liao X, Evans A, He Y (2015) GRETNA: a graph theoretical network analysis toolbox for imaging connectomics. Front Hum Neurosci 9:386
Fornito A, Zalesky A, Bullmore ET (2010) Network scaling effects in graph analytic studies of human resting-state fMRI data. Front Syst Neurosci 4:22
Braun U, Plichta MM, Esslinger C et al (2012) Test-retest reliability of resting-state connectivity network characteristics using fMRI and graph theoretical measures. Neuroimage 59:1404–1412
Zhang J, Wang J, Wu Q et al (2011) Disrupted brain connectivity networks in drug-naive, first-episode major depressive disorder. Biol Psychiatry 70:334–342
Fazekas F, Chawluk JB, Alavi A et al (1987) MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am J Roentgenol 149:351–356
Bournonville C, Hénon H, Dondaine T et al (2018) Identification of a specific functional network altered in poststroke cognitive impairment. Neurology 90:e1879–e1888
Watts DJ, Strogatz SH (1998) Collective dynamics of ‘small-world’ networks. Nature 393:440–442
Sporns O, Chialvo DR, Kaiser M, Hilgetag CC (2004) Organization, development and function of complex brain networks. Trends Cogn Sci 8:418–425
Lynall ME, Bassett DS, Kerwin R et al (2010) Functional connectivity and brain networks in schizophrenia. J Neurosci 30:9477–9487
Sanz-Arigita EJ, Schoonheim MM, Damoiseaux JS et al (2010) Loss of ‘small-world’ networks in Alzheimer’s disease: graph analysis of FMRI resting-state functional connectivity. PLoS One 5:e13788
Zhang J, Liu Z, Li Z et al (2016) Disrupted white matter network and cognitive decline in type 2 diabetes patients. J Alzheimers Dis 53:185–195
Hill CJ, Cardwell CR, Patterson CC et al (2014) Chronic kidney disease and diabetes in the National Health Service: a cross-sectional survey of the U.K. National Diabetes Audit. Diabet Med 31:448–454
Doshi SM, Friedman AN (2017) Diagnosis and management of type 2 diabetic kidney disease. Clin J Am Soc Nephrol 12:1366–1373
Tong L, Adler SG (2018) Diabetic kidney disease. Clin J Am Soc Nephrol 13:335–338
Luo S, Qi RF, Wen JQ et al (2016) Abnormal intrinsic brain activity patterns in patients with end-stage renal disease undergoing peritoneal dialysis: a resting-state functional MR imaging study. Radiology 278(1):181–189
Kong X, Wen JQ, Qi RF et al (2014) Diffuse interstitial brain edema in patients with end-stage renal disease undergoing hemodialysis: a tract-based spatial statistics study. Medicine (Baltimore) 93:e313
Bugnicourt JM, Godefroy O, Chillon JM, Choukroun G, Massy ZA (2013) Cognitive disorders and dementia in CKD: the neglected kidney-brain axis. J Am Soc Nephrol 24:353–363
Zhang Z, Liao W, Chen H et al (2011) Altered functional–structural coupling of large-scale brain networks in idiopathic generalized epilepsy. Brain 134:2912–2928
Zalesky A, Fornito A, Harding IH et al (2010) Whole-brain anatomical networks: does the choice of nodes matter. Neuroimage 50:970–983
Funding
This study was funded by grants from the National Natural Science Foundation of China (81322020, 81230032, 81471644).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Long Jiang Zhang.
Conflict of interest
The authors declare that they have no conflicts of interest.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
Some study subjects or cohorts have not been previously reported.
Methodology
• prospective
• observational
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 63 kb)
Rights and permissions
About this article
Cite this article
Wang, Y.F., Gu, P., Zhang, J. et al. Deteriorated functional and structural brain networks and normally appearing functional–structural coupling in diabetic kidney disease: a graph theory-based magnetic resonance imaging study. Eur Radiol 29, 5577–5589 (2019). https://doi.org/10.1007/s00330-019-06164-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-019-06164-1