Abstract
Objectives
ASL is useful in evaluating tumour blood flow and in detecting hypervascular tumours. The purpose of this study was to assess the additive value of ASL to non-contrast and contrast-enhanced (NC/CE)-T1WI for diagnosing residual or recurrent meningiomas.
Methods
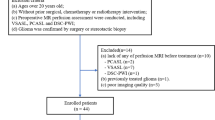
This retrospective study included 25 postoperative patients (20 women, 5 men; median age, 65 [32–85] years) with and 25 gender- and age-matched postoperative patients without residual or recurrent meningiomas. ASL was performed using a pseudocontinuous method. Seven independent observers (two radiology residents, two general radiologists and three neuroradiologists) participated in two reading sessions consisting of only NC/CE-T1WI (first session) or NC/CE-T1WI with ASL (second session). We evaluated the sensitivity and diagnostic performance for the detection of residual or recurrent meningiomas. The diagnostic performance was assessed using a figure of merit (FOM) calculated via jackknife free-response receiver-operating characteristics. Statistical analysis was performed with paired t tests, with a significance level of p < .05.
Results
The sensitivities were as follows (NC/CE-T1WI vs. NC/CE-T1WI with ASL): residents (62.1% vs. 70.7%), general radiologists (75.9% vs. 87.9%), neuroradiologists (97.7% vs. 100%) and all observers (81.3% vs. 88.2%). The FOMs were as follows (NC/CE-T1WI vs. NC/CE-T1WI with ASL): residents (0.76 vs. 0.83), general radiologists (0.83 vs. 0.93), neuroradiologists (0.95 vs. 0.99) and all observers (0.86 vs. 0.93). The addition of ASL significantly improved the diagnostic parameters for all observers except neuroradiologists (p <. 05).
Conclusions
ASL improved the detection rate of residual or recurrent meningiomas on NC/CE-T1WI among both radiology residents and general radiologists.
Key Points
• ASL improved diagnostic performance for residual/recurrent meningioma compare to NC/CE-T1WI alone.
• Diagnostic sensitivity was increased after adding ASL compared with NC/CE-T1WI.
• FOM was increased after adding ASL compared with NC/CE-T1WI.






Similar content being viewed by others
Abbreviations
- ASL:
-
Arterial spin-labeling
- CBF:
-
Cerebral blood flow
- CE-T1WI:
-
Contrast-enhanced T1-weighted imaging
- CNR:
-
Lesion-to-normal contrast-to-noise ratio
- FOM:
-
Figure of merit
- NC-T1WI:
-
Non-contrast T1-weighted imaging
- PCASL:
-
Pseudocontinuous arterial spin-labeling
- PWI:
-
Perfusion-weighted imaging
- TBF:
-
Tumour blood flow
References
Marosi C, Hassler M, Roessler K et al (2008) Meningioma. Crit Rev Oncol Hematol 67:153–171
Louis D, Ohgaki H, Wiestler O et al (2016) WHO classification of tumours of the central nervous system, revised. IARC, Lyon
McKinstry CS, Worthington BS, Niendorf HP, Young IR (1986) Demonstration of meningeal contrast enhancement on magnetic resonance imaging. Acta Radiol Suppl 369:564–567
Toh CH, Wei KC, Chang CN et al (2014) Assessment of angiographic vascularity of meningiomas with dynamic susceptibility contrast-enhanced perfusion-weighted imaging and diffusion tensor imaging. AJNR Am J Neuroradiol 35:263–269
Martin AJ, Cha S, Higashida RT et al (2007) Assessment of vasculature of meningiomas and the effects of embolization with intra-arterial MR perfusion imaging: a feasibility study. AJNR Am J Neuroradiol 28:1771–1777
Tamrazi B, Shiroishi MS, Liu CS (2016) Advanced imaging of intracranial meningiomas. Neurosurg Clin N Am 27:137–143
Mori H, Kunimatsu A, Abe O et al (2012) Diagnostic ability of fluid-attenuated inversion recovery MR imaging to detect remnant or recurrent meningiomas after resection. Neuroradiol J 25:163–171
Kimura H, Takeuchi H, Koshimoto Y et al (2006) Perfusion imaging of meningioma by using continuous arterial spin-labeling: comparison with dynamic susceptibility-weighted contrast-enhanced MR images and histopathologic features. AJNR Am J Neuroradiol 27:85–93
Kawaji H, Koizumi S, Sakai N et al (2013) Evaluation of tumor blood flow after feeder embolization in meningiomas by arterial spin-labeling perfusion magnetic resonance imaging. J Neuroradiol 40:303–306
Simpson D (1957) The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry 20:22–39
Dai W, Garcia D, de Bazelaire C, Alsop DC (2008) Continuous flow-driven inversion for arterial spin labeling using pulsed radio frequency and gradient fields. Magn Reson Med 60:1488–1497
Alsop DC, Detre JA, Golay X et al (2015) Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: a consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med 73:102–116
Kikuchi K, Hiwatashi A, Togao O et al (2017) Correlation between arterial spin-labeling perfusion and histopathological vascular density of pediatric intracranial tumors. J Neurooncol 135:561–569
Wirestam R, Ryding E, Lindgren A et al (2000) Regional cerebral blood flow distributions in normal volunteers: dynamic susceptibility contrast MRI compared with 99mTc-HMPAO SPECT. J Comput Assist Tomogr 24:526–530
Nezafat R, Stuber M, Ouwerkerk R, Gharib AM, Desai MY, Pettigrew RI (2006) B1-insensitive T2 preparation for improved coronary magnetic resonance angiography at 3 T. Magn Reson Med 55:858–864
Kikuchi K, Hiwatashi A, Togao O et al (2015) 3D MR sequence capable of simultaneous image acquisitions with and without blood vessel suppression: utility in diagnosing brain metastases. Eur Radiol 25:901–910
Chakraborty DP, Berbaum KS (2004) Observer studies involving detection and localization: modeling, analysis, and validation. Med Phys 31:2313–2330
Chakraborty DP (2006) Analysis of location specific observer performance data: validated extensions of the jackknife free-response (JAFROC) method. Acad Radiol 13:1187–1193
Zhang H, Rodiger LA, Shen T, Miao J, Oudkerk M (2008) Preoperative subtyping of meningiomas by perfusion MR imaging. Neuroradiology 50:835–840
Koizumi S, Sakai N, Kawaji H et al (2015) Pseudo-continuous arterial spin labeling reflects vascular density and differentiates angiomatous meningiomas from non-angiomatous meningiomas. J Neurooncol 121:549–556
Wang J, Zhang Y, Wolf RL, Roc AC, Alsop DC, Detre JA (2005) Amplitude-modulated continuous arterial spin-labeling 3.0-T perfusion MR imaging with a single coil: feasibility study. Radiology 235:218–228
Maleki N, Dai W, Alsop DC (2012) Optimization of background suppression for arterial spin labeling perfusion imaging. MAGMA 25:127–133
Wu WC, Lin SC, Wang DJ, Chen KL, Li YD (2013) Measurement of cerebral white matter perfusion using pseudocontinuous arterial spin labeling 3T magnetic resonance imaging–an experimental and theoretical investigation of feasibility. PLoS One 8:e82679
Nagao E, Yoshiura T, Hiwatashi A et al (2011) 3D turbo spin-echo sequence with motion-sensitized driven-equilibrium preparation for detection of brain metastases on 3T MR imaging. AJNR Am J Neuroradiol 32:664–670
McCarthy BJ, Davis FG, Freels S et al (1998) Factors associated with survival in patients with meningioma. J Neurosurg 88:831–839
Jääskeläinen J, Haltia M, Servo A (1986) Atypical and anaplastic meningiomas: radiology, surgery, radiotherapy, and outcome. Surg Neurol 25:233–242
Mirimanoff RO, Dosoretz DE, Linggood RM, Ojemann RG, Martuza RL (1985) Meningioma: analysis of recurrence and progression following neurosurgical resection. J Neurosurg 62:18–24
Kanda T, Ishii K, Kawaguchi H, Kitajima K, Takenaka D (2014) High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology 270:834–841
Quattrocchi CC, Mallio CA, Errante Y et al (2015) Gadodiamide and dentate nucleus T1 hyperintensity in patients with meningioma evaluated by multiple follow-up contrast-enhanced magnetic resonance examinations with no systemic interval therapy. Invest Radiol 50:470–472
Acknowledgements
Part of this work was presented at the 54th Annual Meeting of the American Society of Neuroradiology and Foundation of the ASNR Symposium, May 23–26, 2016; Washington, DC., USA: Number O-377
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Hiroshi Honda.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by the institutional review board.
Ethical approval
Institutional review board approval was obtained.
Methodology
• Retrospective
• Diagnostic or prognostic study
Rights and permissions
About this article
Cite this article
Kikuchi, K., Hiwatashi, A., Togao, O. et al. Arterial spin-labeling is useful for the diagnosis of residual or recurrent meningiomas. Eur Radiol 28, 4334–4342 (2018). https://doi.org/10.1007/s00330-018-5404-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-018-5404-4