Abstract
Objectives
Indications for pulmonary valve replacement (PVR) in patients with pulmonary regurgitation (PR) after repaired tetralogy of Fallot (rToF) are debated. We aimed to compare right (RV) and left ventricular (LV) kinetic energy (KE) measured by 4D-flow magnetic resonance imaging (MRI) in patients to controls, to further understand the pathophysiological effects of PR.
Methods
Fifteen patients with rToF with PR > 20% and 14 controls underwent MRI. Ventricular volumes and KE were quantified from cine MRI and 4D-flow, respectively. Lagrangian coherent structures were used to discriminate KE in the PR. Restrictive RV physiology was defined as end-diastolic forward flow.
Results
LV systolic peak KE was lower in rToF, 2.8 ± 1.1 mJ, compared to healthy volunteers, 4.8 ± 1.1 mJ, p < 0.0001. RV diastolic peak KE was higher in rToF (7.7 ± 4.3 mJ vs 3.1 ± 1.3 mJ, p = 0.0001) and the difference most pronounced in patients with non-restrictive RV physiology. KE was primarily located in the PR volume at the time of diastolic peak KE, 64 ± 17%.
Conclusion
This is the first study showing disturbed KE in patients with rToF and PR, in both the RV and LV. The role of KE as a potential early marker of ventricular dysfunction to guide intervention needs to be addressed in future studies.
Key Points
• Kinetic energy (KE) reflects ventricular performance
• KE is a potential marker of ventricular dysfunction in Fallot patients
• KE is disturbed in both ventricles in patients with tetralogy of Fallot
• KE contributes to the understanding of the pathophysiology of pulmonary regurgitation
• Lagrangian coherent structures enable differentiation of ventricular inflows
Similar content being viewed by others
Introduction
Repair of tetralogy of Fallot (rToF) can result in pulmonary regurgitation (PR) which can cause dilatation of the right ventricle (RV), restrictive RV physiology [1], decreased exercise capacity [2] and predisposes the patient to ventricular arrhythmias and sudden death [3]. The indications and timing of pulmonary valve replacement (PVR) in rToF patients with PR are under debate [4,5,6,7]. RV and left ventricular (LV) function are both strongly related to increased mortality, and earlier detection of RV and LV dysfunction may improve selection of patients benefitting from PVR [8].
Four-dimensional (4D)-flow magnetic resonance imaging (MRI) may provide better understanding and early detection of ventricular dysfunction in rToF with PR [9, 10] and the method enables quantification of ventricular kinetic energy (KE) [11]. The KE of blood is defined as the work needed to accelerate a given mass (blood) from rest to its stated velocity. Intracardiac KE has been shown to be disturbed in patients with heart failure, mitral regurgitation and Fontan circulation [12,13,14,15]. However, in patients with rToF, there is only one previously published study, which also did not include analysis of diastolic KE [16]. During diastole, PR and tricuspid inflow both contribute to RV KE. To analyse the KE during diastole these flows can be separated using Lagrangian coherent structures (LCS), as previously shown and validated [17]. Turbulent kinetic energy has been used to study the RV of patients with rToF [18]. Turbulent kinetic energy estimates the amount of energy converted into heat because of viscosity and turbulence. Thus, turbulent KE is a different physical entity showing energy loss, compared to KE that reflects the work needed to accelerate intraventricular blood from rest to the velocity at each time point. KE may help explain the pathophysiology of PR and serve as a biomarker in this disease. The aim of this study was to evaluate ventricular kinetic energy during the entire cardiac cycle in patients with rToF with moderate or severe PR compared to controls, in order to describe the pathophysiological effects of PR on the left and the right ventricles.
Materials and methods
Study design
Fifteen patients, five female, referred for cardiovascular MRI were prospectively included in the study. Inclusion criteria were rToF, with PR > 20% (diagnosed on previous MRI or echocardiography) and no pulmonary stenosis. Age at inclusion was 29 ± 12 years (mean ± SD). Seven of the patients had a systemic to pulmonary shunt prior to the corrective surgery. Median age at correction was 14 months, range 3–350 months (n = 14, age of surgery was uncertain for one patient). Ten patients had a transannular patch inserted. Median time from correction to study inclusion was 18 years (range 9–46 years). One patient had a stenosis of the left pulmonary branch 10 years after corrective surgery, which was treated with a stent 10 years prior to the inclusion. One patient, corrected at age 29, had a reoperation with a conduit 16 years later, 9 years prior to the inclusion. Patients underwent MRI including 4D phase contrast (PC) flow and performed an exercise test with continuous gas analysis to determine peak oxygen uptake (VO2 peak) described in detail below [19]. Six of the patients later underwent pulmonary valve replacement (PVR) and had a follow-up MRI 6–12 months after operation with the same protocol as baseline. Indications for PVR were PR fraction ≥ 35%, progressive RV dilatation with RV end-diastolic volume (EDV) ≥ 150 ml/m2 and/or symptoms and signs of heart failure. Fourteen healthy volunteers, two female, age 30 ± 7 years, were recruited by advertising at the local institution and underwent the same MRI protocol and were used as controls. Inclusion criteria for controls were normal blood pressure (< 140/90 mmHg) and ECG, no cardiovascular medication and no medical history of cardiovascular or other systemic disease.
The principles of the Declaration of Helsinki were followed and the study was approved by the Regional Ethical Review Board, Lund, Sweden. Written informed consent was obtained from all subjects.
Cardiac magnetic resonance imaging
Balanced steady-state free-precession (bSSFP) cine images covering the entire heart were acquired using a 1.5-T Achieva MRI (Philips Healthcare,) or a 1.5-T MAGNETOM Aera MRI (Siemens Healthcare). Two-dimensional (2D) PC flow measurements were performed in the ascending aorta and pulmonary artery to measure the effective stroke volume (SV) and PR. Restrictive RV physiology was defined as end-diastolic forward flow in the pulmonary artery [20, 21]. A PC 4D-flow sequence was used to acquire a three-dimensional (3D) volume covering the whole heart. Typical imaging parameters are reported in Table 1. On both scanners prototype 4D-flow sequences for research purpose were used. The 4D sequences have been previously described and validated in vivo and in vitro [22, 23]. The number of time phases acquired was dependent on heart rate and set to the maximum with a preserved segmentation factor/views per segment of 2. Typical acquired matrix size was 80 × 96 × 35 entries. To minimize the potential effect of using two scanners on the study, nine patients and eight controls were examined with the Philips scanner and six patients and six controls with the Siemens scanner. Follow-up studies after PVR were performed on the same scanner as baseline.
Image analysis and calculation of kinetic energy
Segment software (http://segment.heiberg.se) with an in-house developed module for 4D-flow analysis of KE was used [24]. First-order phase background correction [25] and phase unwrapping was performed. For each voxel the velocity magnitude of the 3D velocity vector was computed.
Delineations of the endocardium were drawn manually over the cardiac cycle in the cine short-axis stack to calculate EDV, end-systolic volume (ESV) and SV. The delineations were transferred to the 4D-flow data set. KE for each voxel was calculated as \( \mathrm{KE}=\frac{\mathrm{m}{\mathrm{v}}^2}{2} \), where m is mass of the voxel, calculated as the density of blood, 1.05 g/cm3, multiplied by the volume of the voxel, and v is the velocity in the voxel. KE over all voxels in the ventricles was calculated for all time phases of the cardiac cycle. Systolic and diastolic peak KE was defined as the highest value during systole and diastole, respectively.
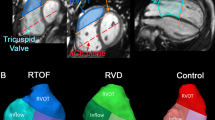
LCS were computed in the short-axis orientation at the time of diastolic peak KE in order to find regions of PR and to calculate how much PR contributed to the KE of the RV, Fig. 1 [17].
a SSFP short-axis view with the right ventricle (RV, outlined in yellow) and the left ventricle (LV) outlined in green. b Same view with Lagrangian coherent structures (LCS) at the time of diastolic peak kinetic energy, visualizing the pulmonary regurgitation (outlined in red) and inflow to the right ventricle. LCS define boundaries of flow fields and thereby give a ring shape when a jet is directed towards or from the viewer in this image plane, e.g. the inflow to the RV
The ratio between systolic/diastolic peaks was calculated to determine if the pattern in the LV and RV differed between patients and controls and if restrictive physiology had an impact on the KE pattern. KE data were linearly interpolated in time to display the average KE over time in patients and controls independent of different heart rates as shown in Fig. 4 [11].
KE was analyzed as absolute values but also indexed to planimetrically calculated SV (EDV-ESV) to be able to compare patients with different heart sizes or valvular insufficiencies and to eliminate the possibility of a larger SV, caused by the PR in rToF patients, explaining potential differences. There have been different ways of indexing KE in earlier publications and therefore we report the values in this study using five different indexing methods.
Exercise performance
Patients performed a maximal exercise test with continuous gas analysis (Carefusion, Oxycon Pro, Jaeger) on a cycle ergometer (939 E, Monark). An individualized protocol with a 1-min rest phase, 2-min reference phase on no or low resistance, followed by a ramp with incremental workload until maximal exhaustion was used. Patients were encouraged to continue until respiratory exchange ratio was 1.1 or more, to ensure maximal exertion. Reference values for VO2 peak were obtained from the SHIP study [19]. Twelve-lead ECG was recorded continuously and blood pressure was obtained every minute.
Statistical analysis
Statistical analysis was performed using GraphPad (v6.04, La Jolla). Values are presented as means ± SD. Differences in KE between rToF patients and healthy volunteers were assessed using the Student t test and differences before and after PVR using paired t test. Cohen’s kappa was analyzed for the relation of systolic/diastolic peak KE and restrictive RV physiology. Correlations were analyzed using Spearman correlation test. Results with a p value less than 0.05 were considered statistically significant.
Results
Results of the volumetric measurements in patients (n = 15, five female, age 29 ± 12 years) and controls (n = 14, two female, age 30 ± 7 years) are shown in Table 2.
Absolute and indexed KE values
Systolic peak KE in the LV was lower in rToF compared to controls. Conversely, in the RV diastolic peak KE was higher than in controls. The differences between rToF and controls remained when indexing for SV, Fig. 2. The absolute and indexed KE values are shown in Table 3 together with different indexations used for KE previously.
The increase in diastolic KE was most pronounced in patients with non-restrictive RV physiology, Fig. 3a, b. This difference was seen even though the degree of PR in patients with restrictive and non-restrictive RV physiology was similar (39 ± 11% vs 39 ± 6%, p = 0.9).
Comparison of systolic and diastolic peak kinetic energy (KE) indexed to stroke volume (SV) in the right ventricle (RV) of patients with repaired tetralogy of Fallot (rToF) with non-restrictive right ventricular physiology, with restrictive right ventricular physiology and controls. Bar and whiskers show mean ± SD. a Results for systolic peak KE/SV with no difference between the groups. b In diastole, rToF has higher KE/SV than controls, but the difference is more pronounced in rToF with non-restrictive right ventricular physiology than with restrictive right ventricular physiology. The bottom panels show two patient examples of kinetic energy (KE) indexed to stroke volume (SV) throughout the cardiac cycle. c Patient with non-restrictive right ventricular (RV) physiology and d patient with restrictive RV physiology. Patients with non-restrictive RV physiology had lower systolic peak KE compared to diastolic peak KE resulting in a ratio of peak systolic to peak diastolic KE of ≤ 1 whereas patients with a restrictive RV physiology had a ratio > 1
There was a moderate positive correlation between systolic peak KE and RVEDV in patients with rToF (R = 0.54, p = 0.04) and a strong positive correlation between diastolic peak KE and RVESV (R = 0.74, p = 0.002). KE was primarily located in the PR volume at the time of diastolic peak KE, 64 ± 17 %, n = 14, and there was a moderate positive correlation between the KE located in the PR fraction and the PR volume, R = 0.56, p = 0.04. Diastolic peak KE in the entire RV, on the other hand, did not correlate with PR volume (p = 0.12).
The differentiation of tricuspid inflow and PR flow is shown in Supplemental Movie 1.
There was no difference (p = 0.20) in the diastolic peak KE in the RV outside the PR volume in rToF (2.5 ± 1.1 mJ, indexed to net SV 0.032 ± 0.014 mJ/ml) and the diastolic peak KE in controls (3.1 ± 1.3 mJ , indexed to SV 0.028 ± 0.01 mJ/ml).
Systolic/diastolic KE ratio
The KE/SV throughout the cardiac cycle in the LV and RV of rToF and controls is shown in Fig. 4. The ratio of systolic/diastolic peak KE was lower in rToF for both the LV (0.5 ± 0.3) and the RV (1.5 ± 0.9), compared to controls (1.0 ± 0.4, p = 0.0031 for LV and 3.0 ± 1.1, p = 0.0006 for RV). Four of five patients with non-restrictive RV physiology had RV systolic/diastolic KE ratio ≤ 1 and the remaining patient had an RV KE ratio of 1.1, whereas a systolic/diastolic KE ratio > 1 was seen in all 10 patients with restrictive RV (Cohen’s kappa 0.84), Fig. 3c, d.
Exercise performance
Mean peak VO2 was 30 ± 10 ml/min/kg (n = 13), equivalent to 86 ± 19 % of the predicted value. There was no correlation between peak VO2 and PR volume, (R = − 0.18, p = 0.56), nor were there any correlations between peak VO2 and systolic or diastolic peak KE/SV in either RV (R = − 0.12, p = 0.70; R = − 0.03, p = 0.92) or LV (R = − 0.19, p = 0.54; R = 0.24, p = 0.44). Furthermore, no correlation was found between peak VO2 and EDV, ESV, SV or EF in either RV (R = − 0.39, p = 0.19; R = − 0.41, p = 0.16; R = − 0.32, p = 0.28; R = 0.16, p = 0.61) or LV (R = − 0.044, p = 0.89; R = − 0.10, p = 0.73; R = 0.047, p = 0.88; R = 0.17, p = 0.59).
Follow-up after pulmonary valve replacement
RVEDV decreased from 316 ± 48 ml to 230 ± 43 ml at follow-up after surgery, RVEDV/BSA from 164 ± 18 ml/m2 to 116 ± 18 ml/m2, RVESV from 188 ± 36 ml to 144 ± 35 ml and RVESV/BSA from 97 ± 3 ml/m2 to 73 ± 15 ml/m2 (n = 6).
Patients after PVR had lower systolic peak KE in both LV and RV, but no difference during diastole, compared to controls, Table 4,
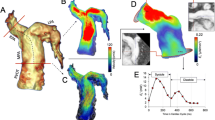
All but one patient had decreased peak KE in the RV after PVR, Fig. 5 and Table 4. Visualizations of KE during systole in the RV before and after PVR in two patients are shown in Fig. 6.
Comparison on individual basis of kinetic energy (KE) and KE indexed to stroke volume (SV) between rToF patients before and after pulmonary valve replacement (PVR). The upper panels show the left ventricle (LV) and the lower panels show the right ventricle (RV). The systolic and the diastolic values are shown in the left and the right column, respectively. All patients but one had a decrease of both systolic and diastolic KE the RV. The rise in KE in one patient is likely explained by the surgical removal of a widening patch in the RV outflow tract at the time of PVR
Visualization of kinetic energy during systole in two patients with tetralogy of Fallot before (left column) and after (right column) pulmonary valve replacement (PVR). Patient 1 shows a decrease in systolic KE in the right ventricle (RV) after operation (b). c, d Patient who had a widening patch in the RVOT removed at the time of PVR, which led to higher systolic KE in the RV after surgery than before
Discussion
This is, to our knowledge, the first study that quantifies KE during the entire cardiac cycle in patients with rToF and PR. Our main findings are (1) Patients with rToF and PR with preserved LV global function have decreased LV systolic peak KE compared to controls. (2) RV diastolic peak KE is increased in patients compared to controls as a result of the KE in the PR volume, but RV systolic KE is not different from controls even though flow volumes are much increased. (3) Patients with restrictive RV physiology have less increased KE compared to patients with non-restrictive RV physiology. (4) After surgical PVR systolic KE was more similar to controls in both ventricles. (5) 4D-flow and LCS enable differentiation of flows in patients with valvular regurgitation.
The decreased systolic peak KE in the LV in patients is interesting as LV strain has been found to be decreased and regional myocardial velocities altered in rToF patients with preserved LVEF [26, 27] and LV function is a strong predictor of outcome in these patients [28]. The difference in KE cannot be attributed to a decreased SV as the difference remains when indexing for SV. The reason for lower systolic peak KE is speculative, but might partly be caused by decreased preload, i.e. an under-filling of the LV due to the RV impairment. This hypothesis is supported by an experimental study that showed that PR leads to decreased longitudinal contribution to RVSV and that the degree of decreased longitudinal contribution to RVSV had a very high correlation with decrease in pulmonary capillary wedge pressure [29]. Septal dyssynchrony with septal movement towards the RV in systole, as described by Stephensen et al. [1] in patients with rToF and also found in the animal model of PR [29], is another factor that may influence the KE in the LV.
The lack of difference in absolute RV systolic peak KE between rToF and controls may seem surprising as the systolic RVSV is higher compared to controls as a result of the PR. KE is determined by mass (volume of blood) and velocity squared. A larger volume passing a fixed RV outflow tract (RVOT) raises KE which explains the functional pressure gradient between the RV and the pulmonary artery seen during increased flows [30]. A possible explanation for our result is that since the RVOT in our group of rToF is wider than in controls a larger volume can pass without increased velocity. Our findings support those of Jeong et al. who found that there was no significant difference between systolic RV KE in rToF compared to healthy volunteers [16]. After PVR there was a non-significant trend toward decreased systolic peak KE in the RV due to the lower volume passing the RVOT. The explanation why the systolic peak KE was even lower compared to the control group postoperatively is presumably the wider RVOT in patients than controls. In one patient systolic peak KE increased after PVR likely because a widening patch in the RVOT was removed at the time of PVR, Fig. 6. Thus, even if the SV postoperatively was lower, the outflow tract in this patient narrowed with increase in velocities causing higher KE.
Diastolic peak KE in the RV was higher in patients than in controls, independent of indexation to SV. Pulmonary regurgitation caused this increase, since the major part of the KE was within the PR volume and the diastolic KE also decreased after PVR.
We showed a positive correlation between RV volumes and KE and this is expected as KE is a product of mass, in this case the RV volume, and the squared velocity of the blood. However, KE and volumes are not simply exchangeable as shown by the lack of correlation between diastolic KE and PR volume. The correlation between PR volume and KE was only demonstrated when separating the KE in the inflowing PR volume.
In the present study, the degree of PR was similar in patients with restrictive and non-restrictive RV physiology, but patients with restrictive RV physiology had lower diastolic KE and different ratio between systolic/diastolic KE compared to non-restrictive patients. The pathophysiological explanation may be that a restrictive RV with less compliance decreases the velocities of the flow through the tricuspid valve and in the PR and thus decreases the KE even if the PR volume is similar. The reasons for systolic to diastolic KE ratio < 1 in a non-restrictive RV and a healthy LV are different. The non-restrictive compliant RV allows high velocities in the PR volume, leading to high diastolic KE, whereas in the healthy LV the high diastolic KE is caused by ventricular suction of blood from the left atrium.
A previous study found that the degree of restrictive physiology had the strongest correlation with predicted VO2 and that RV volumes did not correlate with predicted VO2 [21]. The difference in KE between restrictive and non-restrictive RV physiology is a potential explanation for the differences in exercise capacity. We found a decreased predicted peak VO2 with a large variation, as expected in this patient group [2]. However, no correlation was seen between peak VO2 and KE in either LV or RV, but this may be due to the low number of patients. Larger patient cohorts with 4D-flow examination and measurement of peak VO2 are needed to further investigate this possible relationship.
In this study we adopted a novel approach of using LCS calculated from 4D-flow to differentiate inflow to the RV from the tricuspid valve and the PR. This method can be used in future studies describing the pathophysiology of other valvular regurgitation using KE.
All subjects in this study were investigated at rest. Studies have shown that KE accounts for little of the external work in healthy controls at rest, but the proportion increase to 3% in the LV and 24% in the RV during exercise [31, 32]. What happens to the KE in patients with rToF and PR is not known but the PR has been shown to decrease during exercise [33].
Limitations
The patient population size was rather small, in particular when studying the effect of PVR. Nonetheless, differences between patients and controls were found but future prospective outcome studies are needed to show the clinical impact of these findings.
Conclusion
4D-flow MRI show disturbed kinetic energy in patients with rToF and PR not only in the directly affected RV but also in the LV where the global function is seemingly preserved. 4D-flow KE may be key to understanding why LV function is affected in rToF patients even when global function is normal and explain the strong prognostic impact of LV function in these patients. The clinical importance of adding 4D-flow data to an echocardiographic examination and in particular if the information from 4D-flow alters clinical decision-making and outcome will be of interest in future studies.
Abbreviations
- 3D:
-
Three dimensional
- 4D:
-
Four dimensional
- CO:
-
Cardiac output
- EDV:
-
End-diastolic volume
- ESV:
-
End-systolic volume
- KE:
-
Kinetic energy
- LCS:
-
Lagrangian coherent structures
- LV:
-
Left ventricle
- m :
-
Mass
- MRI:
-
Magnetic resonance imaging
- PC:
-
Phase contrast
- PR:
-
Pulmonary regurgitation
- PVR:
-
Pulmonary valve replacement
- rToF:
-
Repair of tetralogy of Fallot
- RV:
-
Right ventricle
- RVOT:
-
Right ventricular outflow tract
- SV:
-
Stroke volume
- v :
-
Velocity
- VO2 peak:
-
Peak oxygen uptake
References
Stephensen S, Steding-Ehrenborg K, Munkhammar P et al (2014) The relationship between longitudinal, lateral, and septal contribution to stroke volume in patients with pulmonary regurgitation and healthy volunteers. Am J Physiol Heart Circ Physiol 306:H895–H903
Kempny A, Dimopoulos K, Uebing A et al (2012) Reference values for exercise limitations among adults with congenital heart disease. Relation to activities of daily life–single centre experience and review of published data. Eur Heart J 33:1386–1396
Harrison DA, Harris L, Siu SC et al (1997) Sustained ventricular tachycardia in adult patients late after repair of tetralogy of Fallot. J Am Coll Cardiol 30:1368–1373
Bokma JP, Winter MM, Oosterhof T et al (2015) Preoperative thresholds for mid-to-late haemodynamic and clinical outcomes after pulmonary valve replacement in tetralogy of Fallot. Eur Heart J 37:1–7
Alvarez-Fuente M, Garrido-Lestache E, Fernandez-Pineda L et al (2016) Timing of pulmonary valve replacement: how much can the right ventricle dilate before it looses its remodeling potential? Pediatr Cardiol 37:601–605
Shin YR, Jung JW, Kim NK et al (2016) Factors associated with progression of right ventricular enlargement and dysfunction after repair of tetralogy of Fallot based on serial cardiac magnetic resonance imaging. Eur J Cardiothorac Surg 50:464–469
Greutmann M (2016) Tetralogy of Fallot, pulmonary valve replacement, and right ventricular volumes: are we chasing the right target? Eur Heart J 37:836–839
Bokma JP, de Wilde KC, Vliegen HW et al (2017) Value of cardiovascular magnetic resonance imaging in noninvasive risk stratification in tetralogy of Fallot. JAMA Cardiol 119:1370–1377
Lee N, Taylor MD, Banerjee RK (2015) Right ventricle-pulmonary circulation dysfunction: a review of energy-based approach. Biomed Eng Online 14:S8
Hirtler D, Garcia J, Barker AJ, Geiger J (2016) Assessment of intracardiac flow and vorticity in the right heart of patients after repair of tetralogy of Fallot by flow-sensitive 4D MRI. Eur Radiol 26:3598–3607
Arvidsson PM, Töger J, Heiberg E et al (2013) Quantification of left and right atrial kinetic energy using four-dimensional intracardiac magnetic resonance imaging flow measurements. J Appl Physiol 114:1472–1481
Eriksson J, Bolger AF, Ebbers T, Carlhäll C-JJ (2013) Four-dimensional blood flow-specific markers of LV dysfunction in dilated cardiomyopathy. Eur Heart J Cardiovasc Imaging 14:417–424
Al-Wakeel N, Fernandes JF, Amiri A et al (2015) Hemodynamic and energetic aspects of the left ventricle in patients with mitral regurgitation before and after mitral valve surgery. J Magn Reson Imaging 42:1705–1712
Kanski M, Arvidsson PM, Töger J et al (2015) Left ventricular fluid kinetic energy time curves in heart failure from cardiovascular magnetic resonance 4D flow data. J Cardiovasc Magn Reson 17:111
Sjöberg P, Heiberg E, Wingren P et al (2017) Decreased diastolic ventricular kinetic energy in young patients with Fontan circulation demonstrated by four-dimensional cardiac magnetic resonance imaging. Pediatr Cardiol 38:669–680
Jeong D, Anagnostopoulos PV, Roldan-Alzate A et al (2014) Ventricular kinetic energy may provide a novel noninvasive way to assess ventricular performance in patients with repaired tetralogy of Fallot. J Thorac Cardiovasc Surg 149:1339–1347
Töger J, Kanski M, Carlsson M et al (2012) Vortex ring formation in the left ventricle of the heart: analysis by 4D flow MRI and Lagrangian coherent structures. Ann Biomed Eng 40:1–11
Fredriksson A, Trzebiatowska-Krzynska A, Dyverfeldt P et al (2017) Turbulent kinetic energy in the right ventricle: potential MR marker for risk stratification of adults with repaired tetralogy of Fallot. J Magn Reson Imaging. https://doi.org/10.1002/jmri.25830
Gläser S, Koch B, Ittermann T et al (2010) Influence of age, sex, body size, smoking, and β blockade on key gas exchange exercise parameters in an adult population. Eur J Cardiovasc Prev Rehabil 17:469–476
Munkhammar P, Carlsson M, Arheden H, Pesonen E (2013) Restrictive right ventricular physiology after tetralogy of Fallot repair is associated with fibrosis of the right ventricular outflow tract visualized on cardiac magnetic resonance imaging. Eur Heart J Cardiovasc Imaging 14:978–985
Lee W, Yoo S-J, Roche SL et al (2013) Determinants and functional impact of restrictive physiology after repair of tetralogy of Fallot: new insights from magnetic resonance imaging. Int J Cardiol 167:1347–1353
Kanski M, Töger J, Steding-Ehrenborg K et al (2015) Whole-heart four-dimensional flow can be acquired with preserved quality without respiratory gating, facilitating clinical use: a head-to-head comparison. BMC Med Imaging 15:20
Carlsson M, Bock J, Bidhult S et al (2017) Multi-vendor whole-heart 4D flow validation study with and without respiratory gating. In: 20th Annu SCMR Sci Sess. Abstr Suppl p P327
Heiberg E, Sjögren J, Ugander M et al (2010) Design and validation of Segment—freely available software for cardiovascular image analysis. BMC Med Imaging 10:1
Busch J, Giese D, Kozerke S (2017) Image-based background phase error correction in 4D flow MRI revisited. J Magn Reson Imaging 1–10
Ordovas KG, Carlsson M, Lease KE et al (2012) Impaired regional left ventricular strain after repair of tetralogy of Fallot. J Magn Reson Imaging 35:79–85
Chang M-C, Wu M-T, Weng K-P et al (2018) Left ventricular regional myocardial motion and twist function in repaired tetralogy of Fallot evaluated by magnetic resonance tissue phase mapping. Eur Radiol 28:104–114
Diller G-PP, Kempny A, Liodakis E et al (2012) Left ventricular longitudinal function predicts life-threatening ventricular arrhythmia and death in adults with repaired tetralogy of Fallot. Circulation 125:2440–2446
Kopic S, Stephensen SS, Heiberg E et al (2017) Isolated pulmonary regurgitation causes decreased right ventricular longitudinal function and compensatory increased septal pumping in a porcine model. Acta Physiol 221:163–173
Rao PS, Awa S, Linde LM et al (1973) Role of kinetic energy in pulmonary valvar pressure gradients. Circulation 48:65–73
Carlsson M, Heiberg E, Toger J, Arheden H (2012) Quantification of left and right ventricular kinetic energy using four-dimensional intracardiac magnetic resonance imaging flow measurements. Am J Physiol Heart Circ Physiol 302:H893–H900
Prec O, Katz N, Sennet L et al (1949) Determination of kinetic energy of the heart in man. Am J Physiol 159:483–491
Barber NJ, Ako EO, Kowalik GT et al (2016) Magnetic resonance-augmented cardiopulmonary exercise testing. Circ Cardiovasc Imaging 9:e005282
Acknowledgements
Philips Healthcare is gratefully acknowledged for a research collaboration making the 4D-flow sequence available. We thank Siemens Healthcare for providing the 4D-flow sequence as the work-in-progress package WIP785K.
Funding
This study has received funding by Swedish Heart–Lung foundation, Lund University; Skane University Hospital, Region of Skane; Swedish Research Council and Swedish Medical Association. These funding bodies had no influence over study design, analysis or data interpretation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Marcus Carlsson.
Conflict of interest
The authors of this manuscript declare relationships with the following companies: Einar Heiberg is stockholder and founder of Medviso AB that sells the Segment software for clinical use. Hakan Arheden is stockholder of Imacor AB, a core lab for medical image analysis. Marcus Carlsson has received consultancy fees from Imacor AB. The remaining authors have no competing interests.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional review board approval was obtained.
Methodology
• prospective
• case–control study
• performed at one institution
Electronic supplementary material
ESM 1
(MP4 550 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Sjöberg, P., Bidhult, S., Bock, J. et al. Disturbed left and right ventricular kinetic energy in patients with repaired tetralogy of Fallot: pathophysiological insights using 4D-flow MRI. Eur Radiol 28, 4066–4076 (2018). https://doi.org/10.1007/s00330-018-5385-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-018-5385-3