Abstract
Objectives
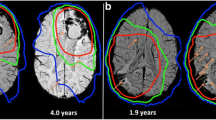
To evaluate the prevalence of cerebral remote microhaemorrhages (RMH) and remote haematomas (RH) using magnetic resonance susceptibility-weighted imaging (SWI) among patients treated for gliomas during follow-up.
Methods
We conducted a retrospective single centre longitudinal study on 58 consecutive patients treated for gliomas from January 2009 through December 2010. Our institutional review board approved this study. We evaluated the presence and number of RMH and RH found outside the brain tumour on follow-up MR imaging. We performed univariate and bivariate analyses to identify predictors for RMH and RH and Kaplan–Meier survival analysis techniques.
Results
Twenty-five (43%) and four patients (7%) developed at least one RMH or RH, respectively, during follow-up. The risk was significantly higher for patients who received radiation therapy (49% and 8% versus 0%) (p = 0.02). The risk of developing RH was significantly higher in patients with at least one RMH and a high burden of RMH. The mean age of those presenting with at least one RMH or RH was significantly lower.
Conclusions
RMH were common in adult survivors of gliomas who received radiation therapy and may predict the onset of RH during follow-up, mainly in younger patients.
Key Points
• Brain RMH and RH are significantly more likely to occur after RT.
• RMH occur in almost half of the patients treated with RT.
• RMH and RH are significantly more frequent in younger patients.
• RH occur only in patients with RMH.


Similar content being viewed by others
Abbreviations
- MH:
-
Microhaemorrhage
- MR:
-
Magnetic resonance
- RH:
-
Remote haematoma
- RMH:
-
Remote microhaemorrhage
- RT:
-
Radiation therapy
- SWI:
-
Susceptibility-weighted imaging
References
Shinohara C, Muragaki Y, Maruyama T et al (2008) Long-term prognostic assessment of 185 newly diagnosed gliomas: grade III glioma showed prognosis comparable to that of Grade II glioma. Jpn J Clin Oncol 38:730–733
Stupp R, Mason WP, van den Bent MJ et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996
Douw L, Klein M, Fagel SS et al (2009) Cognitive and radiological effects of radiotherapy in patients with low-grade glioma: long-term follow-up. Lancet Neurol 8:810–8182
Peters S, Pahl R, Claviez A, Jansen O (2013) Detection of irreversible changes in susceptibility-weighted images after whole-brain irradiation of children. Neuroradiology 55:853–859
Lupo JM, Chuang CF, Chang SM et al (2012) 7-Tesla susceptibility-weighted imaging to assess the effects of radiotherapy on normal-appearing brain in patients with glioma. Int J Radiat Oncol Biol Phys 82:e493–e500
Jain R, Robertson PL, Gandhi D et al (2005) Radiation-induced cavernomas of the brain. AJNR Am J Neuroradiol 26:1158–1162
Bian W, Hess CP, Chang SM et al (2014) Susceptibility-weighted MR imaging of radiation therapy-induced cerebral microbleeds in patients with glioma: a comparison between 3T and 7T. Neuroradiology 56:91–96
Zeng Q-S, Kang X-S, Li C-F, Zhou G-Y (2011) Detection of hemorrhagic hypointense foci in radiation injury region using susceptibility-weighted imaging. Acta Radiol 52:115–119
Lupo JM, Molinaro AM, Essock-Burns E et al (2016) The effects of anti-angiogenic therapy on the formation of radiation-induced microbleeds in normal brain tissue of patients with glioma. Neuro Oncol 18:87–95
Walker MD, Alexander E, Hunt WE et al (1978) Evaluation of BCNU and/or radiotherapy in the treatment of anaplastic gliomas. A cooperative clinical trial. J Neurosurg 49:333–343
Valk PE, Dillon WP (1991) Radiation injury of the brain. AJNR Am J Neuroradiol 12:45–62
Roddy E, Sear K, Felton E et al (2016) Presence of cerebral microbleeds is associated with worse executive function in pediatric brain tumor survivors. Neuro-Oncol 18:1548–1558
Charidimou A, Werring DJ (2012) Cerebral microbleeds and cognition in cerebrovascular disease: an update. J Neurol Sci 322:50–55
Valenti R, Del Bene A, Poggesi A et al (2016) Cerebral microbleeds in patients with mild cognitive impairment and small vessel disease: The Vascular Mild Cognitive Impairment (VMCI)-Tuscany study. J Neurol Sci 368:195–202
Ayaz M, Boikov AS, Haacke EM et al (2010) Imaging cerebral microbleeds using susceptibility weighted imaging: one step toward detecting vascular dementia. J Magn Reson Imaging JMRI 31:142–148
Werring DJ, Gregoire SM, Cipolotti L (2010) Cerebral microbleeds and vascular cognitive impairment. J Neurol Sci 299:131–135
Landis JR, Koch GG (1977) An application of hierarchical kappa-type statistics in the assessment of majority agreement among multiple observers. Biometrics 33:363–374
Core Team R (2014) R: A language and environment for statistical computing. In: R Foundation for Statistical Computing, Vienna http://www.R-project.org/. Accessed date: 2014
Reinhold HS, Hopewell JW (1980) Late changes in the architecture of blood vessels of the rat brain after irradiation. Br J Radiol 53:693–696
Cutsforth-Gregory JK, Lanzino G, Link MJ et al (2015) Characterization of radiation-induced cavernous malformations and comparison with a nonradiation cavernous malformation cohort. J Neurosurg 122:1214–1222
Smart D (2017) Radiation toxicity in the central nervous system: mechanisms and strategies for injury reduction. Semin Radiat Oncol 27:332–339
Vinchon M, Leblond P, Caron S et al (2011) Radiation-induced tumors in children irradiated for brain tumor: a longitudinal study. Childs Nerv Syst 27:445–453
Mirimanoff R-O, Gorlia T, Mason W et al (2006) Radiotherapy and temozolomide for newly diagnosed glioblastoma: recursive partitioning analysis of the EORTC 26981/22981-NCIC CE3 phase III randomized trial. J Clin Oncol 24:2563–2569
Cairncross G, Wang M, Shaw E et al (2013) Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma: long-term results of RTOG 9402. J Clin Oncol 31:337–343
Ducray F, Idbaih A (2013) Neuro-oncology: anaplastic oligodendrogliomas-value of early chemotherapy. Nat Rev Neurol 9:7–8
Jastaniyah N, Murtha A, Pervez N et al (2013) Phase I study of hypofractionated intensity modulated radiation therapy with concurrent and adjuvant temozolomide in patients with glioblastoma multiforme. Radiat Oncol Lond Engl 8:38
Combs SE (2017) Does proton therapy have a future in CNS tumors? Curr Treat Options Neurol 19:12
Floyd NS, Woo SY, Teh BS et al (2004) Hypofractionated intensity-modulated radiotherapy for primary glioblastoma multiforme. Int J Radiat Oncol Biol Phys 58:721–726
Chen Y-D, Feng J, Fang T et al (2013) Effect of intensity-modulated radiotherapy versus three-dimensional conformal radiotherapy on clinical outcomes in patients with glioblastoma multiforme. Chin Med J (Engl) 126:2320–2324
Zach L, Stall B, Ning H et al (2009) A dosimetric comparison of four treatment planning methods for high grade glioma. Radiat Oncol Lond Engl 4:45
Letarte N, Bressler LR, Villano JL (2013) Bevacizumab and central nervous system (CNS) hemorrhage. Cancer Chemother Pharmacol 71:1561–1565
Zuo P-Y, Chen X-L, Liu Y-W et al (2014) Increased risk of cerebrovascular events in patients with cancer treated with bevacizumab: a meta-analysis. PLoS One 9:e102484
Hapani S, Sher A, Chu D, Wu S (2010) Increased risk of serious hemorrhage with bevacizumab in cancer patients: a meta-analysis. Oncology 79:27–38
Kamba T, McDonald DM (2007) Mechanisms of adverse effects of anti-VEGF therapy for cancer. Br J Cancer 96:1788–1795
de Souza JM, Domingues RC, Cruz LCH et al (2008) Susceptibility-weighted imaging for the evaluation of patients with familial cerebral cavernous malformations: a comparison with t2-weighted fast spin-echo and gradient-echo sequences. AJNR Am J Neuroradiol 29:154–158
de Champfleur NM, Langlois C, Ankenbrandt WJ et al (2011) Magnetic resonance imaging evaluation of cerebral cavernous malformations with susceptibility-weighted imaging. Neurosurgery 68:641–647 discussion 647-648
Bian W, Banerjee S, Kelly DAC et al (2015) Simultaneous imaging of radiation-induced cerebral microbleeds, arteries and veins, using a multiple gradient echo sequence at 7 Tesla. J Magn Reson Imaging JMRI 42:269–279
Jeerakathil T, Wolf PA, Beiser A et al (2004) Cerebral microbleeds: prevalence and associations with cardiovascular risk factors in the Framingham Study. Stroke 35:1831–1835
Tsushima Y, Tanizaki Y, Aoki J, Endo K (2002) MR detection of microhemorrhages in neurologically healthy adults. Neuroradiology 44:31–36
Acknowledgements
Laura McMaster provided professional English-language medical editing of this article.
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Augustin Lecler.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors has significant statistical expertise.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Our institutional review board approved this study and waived the informed consent requirement due to its retrospective nature.
Methodology
• retrospective
• observational study
• performed at one institution
Electronic supplementary material
ESM 1
(DOCX 742 kb)
Rights and permissions
About this article
Cite this article
Lecler, A., Charbonneau, F., Psimaras, D. et al. Remote brain microhaemorrhages may predict haematoma in glioma patients treated with radiation therapy. Eur Radiol 28, 4324–4333 (2018). https://doi.org/10.1007/s00330-018-5356-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-018-5356-8