Abstract
Objectives
To determine the feasibility of balanced steady-state free precession (b-SSFP) imaging for measuring hepatic steatosis in obese children and adolescents, using proton magnetic resonance spectroscopy (1H MRS) as reference standard.
Methods
182 obese Chinese paediatric patients underwent conventional T1-weighted dual echo MRI, 1H MRS and b-SSFP imaging for non-invasive assessment of hepatic steatosis.
Results
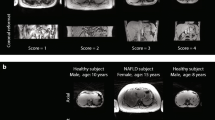
There was a strong positive correlation between liver fat fraction (FF) on T1-weighted dual echo MRI and 1H MRS-determined liver fat content (LFC) (r = 0.964, p < .001), and a strong negative correlation between the ratio of liver signal intensity (SI) to spleen SI (L/S) on b-SSFP and LFC (r = −0.896, p < .001). ROC curve analysis based on a diagnostic threshold of 1H MRS-determined LFC >50 mg/g (>5 % by wet weight) showed areas under the curves for FF and L/S at 0.989 (0.976–1.000) and 0.926 (0.888–0.964), respectively. Optimal FF and L/S cut-off values identified patients with hepatic steatosis with 97.9 % and 86.5 % sensitivity and 93.4 % and 93.4 % specificity, respectively.
Conclusions
Following further validation, b-SSFP at 1.5T has potential as a feasible technique for evaluation of hepatic steatosis in obese paediatric patients with limited breath-holding capacity.
Key Points
• L/S on b-SSFP images closely correlated with 1 H MRS-determined LFC.
• b-SSFP has high diagnostic accuracy for hepatic steatosis in obese children.
• 100% of obese paediatric subjects are imaged successfully using b-SSFP sequence.
• b-SSFP has potential to evaluate hepatic steatosis in children with poor breath-hold.




Similar content being viewed by others
Abbreviations
- 1H MRS:
-
Proton magnetic resonance spectroscopy
- 2D:
-
Two dimensional
- AUC:
-
Area under the curve
- b-SSFP:
-
Balanced steady-state free precession
- BMI:
-
Body mass index
- FF:
-
Fat fraction
- GRE:
-
Gradient recalled echo
- HOMA-IR:
-
Homeostasis model assessment of insulin resistance
- IP:
-
In phase
- L/S:
-
Liver signal intensity (SI) to spleen SI
- LFC:
-
Liver fat content
- MRI:
-
Magnetic resonance imaging
- NAFLD:
-
Non-alcoholic fatty liver disease
- OP:
-
Out-of-phase
- ROC:
-
Receiver operating characteristic
- ROI:
-
Regions of interest
- SI:
-
Signal intensity
- TE:
-
Echo time
- TR:
-
Repetition time
References
Ciocca M, Ramonet M, Alvarez F (2016) Non-alcoholic fatty liver disease: a new epidemic in children. Arch Argent Pediatr 114(6):563–569
Clemente MG, Mandato C, Poeta M, Vajro P (2016) Pediatric non-alcoholic fatty liver disease: Recent solutions, unresolved issues, and future research directions. World J Gastroenterol 22(36):8078–8093
Schwimmer JB, Newton KP, Awai HI et al (2013) Paediatric gastroenterology evaluation of overweight and obese children referred from primary care for suspected non-alcoholic fatty liver disease. Aliment Pharmacol Ther 38(10):1267–1277
Kleiner DE, Makhlouf HR (2016) Histology of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis in Adults and Children. Clin Liver Dis 20(2):293–312
Adams LA, Waters OR, Knuiman MW, Elliott RR, Olynyk JK (2009) NAFLD as a risk factor for the development of diabetes and the metabolic syndrome: an eleven-year follow-up study. Am J Gastroenterol 104(4):861–867
Zhang H, Yang H, Lai C, Xu X, Huang K, Fu J (2015) Quantitative relationship between liver fat content and metabolic syndrome in obese children and adolescents. Clin Endocrinol (Oxf) 83(1):43–49
Pacifico L, Chiesa C, Anania C et al (2014) Nonalcoholic fatty liver disease and the heart in children and adolescents. World J Gastroenterol 20(27):9055–9071
Pais R, Giral P, Khan JF et al (2016) Fatty liver is an independent predictor of early carotid atherosclerosis. J Hepatol 65(1):95–102
van Werven JR, Marsman HA, Nederveen AJ et al (2010) Assessment of hepatic steatosis in patients undergoing liver resection: comparison of US, CT, T1-weighted dual-echo MR imaging, and point-resolved 1H MR spectroscopy. Radiology 256(1):159–168
Bohte AE, van Werven JR, Bipat S, Stoker J (2011) The diagnostic accuracy of US, CT, MRI and 1H-MRS for the evaluation of hepatic steatosis compared with liver biopsy: a meta-analysis. Eur Radiol 21(1):87–97
Shih KL, Su WW, Chang CC et al (2016) Comparisons of parallel potential biomarkers of 1H-MRS-measured hepatic lipid content in patients with non-alcoholic fatty liver disease. Sci Rep 6:24031
Bril F, Barb D, Portillo-Sanchez P et al (2017) Metabolic and histological implications of intrahepatic triglyceride content in nonalcoholic fatty liver disease. Hepatology 65(4):1132–1144
Siegelman ES, Rosen MA (2001) Imaging of hepatic steatosis. Semin Liver Dis 21(1):71–80
Yokoo T, Bydder M, Hamilton G et al (2009) Nonalcoholic fatty liver disease: diagnostic and fat-grading accuracy of low-flip-angle multiecho gradient-recalled-echo MR imaging at 1.5 T. Radiology 251(1):67–76
Glockner JF, Lee CU (2014) Balanced steady state-free precession (b-SSFP) imaging for MRCP: techniques and applications. Abdom Imaging 39(6):1309–1322
Knobloch G, Lauff M-T, Hirsch S, Schwenke C, Hamm B, Wagner M (2016) Nonenhanced magnetic resonance angiography (MRA) of the calf arteries at 3 Tesla: intraindividual comparison of 3D flow-dependent subtractive MRA and 2D flow-independent non-subtractive MRA. Eur Radiol 26(12):4585–4594
Shigenaga Y, Okajima K, Ikeuchi K et al (2016) Usefulness of non-contrast-enhanced MRI with two-dimensional balanced steady-state free precession for the acquisition of the pulmonary venous and left atrial anatomy pre catheter ablation of atrial fibrillation: Comparison with contrast enhanced CT in clinical cases. J Magn Reson Imaging 43(2):495–503
Serai S, Towbin AJ, Podberesky DJ (2012) Non-contrast MRA using an inflow-enhanced, inversion recovery SSFP technique in pediatric abdominal imaging. Pediatr Radiol 42(3):364–368
Chavhan GB, Shelmerdine S, Jhaveri K, Babyn PS (2016) Liver MR Imaging in Children: Current Concepts and Technique. Radiographics 36(5):1517–1532
Li H, Ji CY, Zong XN, Zhang YQ (2009) Body mass index growth curves for Chinese children and adolescents aged 0 to 18 years. Zhonghua Er Ke Za Zhi 47(7):493–498
Zhang HX, Xu XQ, Fu JF, Lai C, Chen XF (2015) Predicting hepatic steatosis and liver fat content in obese children based on biochemical parameters and anthropometry. Pediatr Obes 10(2):112–117
Cassidy FH, Yokoo T, Aganovic L et al (2009) Fatty liver disease: MR imaging techniques for the detection and quantification of liver steatosis. Radiographics 29(1):231–260
Kotronen A, Peltonen M, Hakkarainen A et al (2009) Prediction of non-alcoholic fatty liver disease and liver fat using metabolic and genetic factors. Gastroenterology 137(3):865–872
Longo R, Pollesello P, Ricci C et al (1995) Proton MR spectroscopy in quantitative in vivo determination of fat content in human liver steatosis. J Magn Reson Imaging 5(3):281–285
Szczepaniak LS, Nurenberg P, Leonard D et al (2005) Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab 288(2):E462–E468
Kukuk GM, Hittatiya K, Sprinkart AM et al (2015) Comparison between modified Dixon MRI techniques, MR spectroscopic relaxometry, and different histologic quantification methods in the assessment of hepatic steatosis. Eur Radiol 25(10):2869–2879
Guaraldi G, Besutti G, Stentarelli C et al (2012) Magnetic resonance for quantitative assessment of liver steatosis: a new potential tool to monitor antiretroviral-drug-related toxicities. Antivir Ther 17(6):965–971
Di Martino M, Pacifico L, Bezzi M et al (2016) Comparison of magnetic resonance spectroscopy, proton density fat fraction and histological analysis in the quantification of liver steatosis in children and adolescents. World J Gastroenterol 22(39):8812–8819
Rehm JL, Wolfgram PM, Hernando D, Eickhoff JC, Allen DB, Reeder SB (2015) Proton density fat-fraction is an accurate biomarker of hepatic steatosis in adolescent girls and young women. Eur Radiol 25(10):2921–2930
Heba ER, Desai A, Zand KA et al (2016) Accuracy and the effect of possible subject-based confounders of magnitude-based MRI for estimating hepatic proton density fat fraction in adults, using MR spectroscopy as reference. J Magn Reson Imaging 43(2):398–406
Lim RP, Tuvia K, Hajdu CH et al (2010) Quantification of hepatic iron deposition in patients with liver disease: comparison of chemical shift imaging with single-echo T2*-weighted imaging. AJR Am J Roentgenol 194(5):1288–1295
Sebastiani G, Tempesta D, Alberti A (2012) Hepatic iron overload is common in chronic hepatitis B and is more severe in patients coinfected with hepatitis D virus. J Viral Hepat 19(2):e170–e176
Kuhn JP, Meffert P, Heske C et al (2017) Prevalence of Fatty Liver Disease and Hepatic Iron Overload in a Northeastern German Population by Using Quantitative MR Imaging. Radiology 284(3):706–716
Manco M, Alisi A, Fernandez Real JM et al (2011) Early interplay of intra-hepatic iron and insulin resistance in children with non-alcoholic fatty liver disease. J Hepatol 55(3):647–653
McPherson S, Jonsson JR, Cowin GJ et al (2009) Magnetic resonance imaging and spectroscopy accurately estimate the severity of steatosis provided the stage of fibrosis is considered. J Hepatol 51(2):389–397
Acknowledgements
The authors thank Prof. Jun-fen Fu as the scientific guarantor, and Prof. Yun-xian Yu from the Department of Epidemiology and Health Statistics School of Public Health, Zhejiang University, for his data analysis and assistance with statistics.
Funding
This study has received funding by the National Natural Science Foundations of China (Grant No. 81270938 and NO. 81570759), Zhejiang provincial key disciplines of medicine (Innovation discipline, 11-CX24), and Scientific Research Fund of Zhejiang Provincial Education Department (N20140120).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Prof. Jun-fen Fu.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
Prof. Yun-xian Yu from the Department of Epidemiology and Health Statistics School of Public Health, Zhejiang University, for his data analysis and assistance with statistics.
Informed consent
Written informed consent was obtained from all children and adolescents and/or their guardians in this study.
Ethical approval
Ethical approval for this study was obtained from the Medical Committee of the Children's Hospital Zhejiang University School of Medicine, China.
Methodology
• prospective
• cross sectional study / diagnostic or prognostic study
• performed at one institution
Electronic supplementary material
ESM 1
(DOCX 26 kb)
Rights and permissions
About this article
Cite this article
Zhang, Hx., Fu, Jf., Lai, C. et al. Feasibility of balanced steady-state free precession sequence at 1.5T for the evaluation of hepatic steatosis in obese children and adolescents. Eur Radiol 28, 4479–4487 (2018). https://doi.org/10.1007/s00330-018-5344-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-018-5344-z