Abstract
Objectives
To identify disease-related spatial covariance patterns of grey matter volume as an aid in the classification of Parkinson’s disease (PD).
Methods
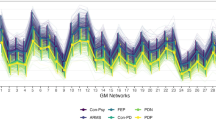
Seventy structural covariance networks (SCNs) based on grey matter volume covariance patterns were defined using independent component analysis with T1-weighted structural MRI scans (discovery sample, 70 PD patients and 70 healthy controls). An image-based classifier was constructed from SCNs using a multiple logistic regression analysis with a leave-one-out cross-validation-based feature selection scheme. A validation sample (26 PD patients and 26 healthy controls) was further collected to evaluate the generalization ability of the constructed classifier.
Results
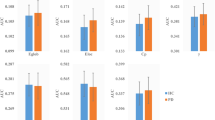
In the discovery sample, 13 SCNs, including the cerebellum, anterior temporal poles, parahippocampal gyrus, parietal operculum, occipital lobes, supramarginal gyri, superior parietal lobes, paracingulate gyri and precentral gyri, had higher classification performance for PD. In the validation sample, the classifier had moderate generalization ability, with a mean sensitivity of 81%, specificity of 69% and overall accuracy of 75%. Furthermore, certain individual SCNs were also associated with disease severity.
Conclusions
Although not applicable for routine care at present, our results provide empirical evidence that disease-specific, large-scale structural networks can provide a foundation for the further improvement of diagnostic MRI in movement disorders.
Key Points
• Disease-specific, large-scale SCNs can be identified from structural MRI.
• A new network-based framework for PD classification is proposed.
• An SCN-based classifier had moderate generalization ability in PD classification.
• The selected SCNs provide valuable functional information regarding PD patients.



Similar content being viewed by others
Abbreviations
- ANCOVA:
-
Analysis of covariance
- CSF:
-
Cerebrospinal fluid
- DARTEL:
-
Diffeomorphic anatomical registration exponentiated lie algebra
- GMV:
-
Grey matter volume
- HY stage:
-
Hoehn and Yahr stages
- ICA:
-
Independent component analysis
- LOOCV:
-
Leave-one-out cross-validation
- MNI:
-
Montreal Neurological Institute
- MELODIC:
-
Multivariate exploratory linear optimized decomposition into independent components
- PD:
-
Parkinson’s disease
- ROC:
-
Receiver operator characteristic
- SE-ADL:
-
Schwab and England activities of daily living scale
- SCNs:
-
Structural covariance networks
- UPDRS:
-
Unified Parkinson’s disease rating scale
- VBM:
-
Voxel-based morphometry
- WM:
-
White matter
References
Sveinbjornsdottir S (2016) The clinical symptoms of Parkinson's disease. J Neurochem 139:318–324
Chaudhuri KR, Healy DG, Schapira AH, National Institute for Clinical Excellence (2006) Non-motor symptoms of Parkinson's disease: diagnosis and management. Lancet Neurol 5:235–245
Luo C, Song W, Chen Q et al (2014) Reduced functional connectivity in early-stage drug-naive Parkinson's disease: a resting-state fMRI study. Neurobiol Aging 35:431–441
Pan PL, Song W, Shang HF (2012) Voxel-wise meta-analysis of gray matter abnormalities in idiopathic Parkinson's disease. Eur J Neurol 19:199–206
Tessitore A, Amboni M, Cirillo G et al (2012) Regional gray matter atrophy in patients with Parkinson disease and freezing of gait. AJNR Am J Neuroradiol 33:1804–1809
Tessitore A, Santangelo G, De Micco R et al (2016) Cortical thickness changes in patients with Parkinson's disease and impulse control disorders. Parkinsonism Relat Disord 24:119–125
Mak E, Su L, Williams GB et al (2015) Baseline and longitudinal grey matter changes in newly diagnosed Parkinson's disease: ICICLE-PD study. Brain 138:2974–2986
Mechelli A, Friston KJ, Frackowiak RS, Price CJ (2005) Structural covariance in the human cortex. J Neurosci 25:8303–8310
Chou KH, Lin WC, Lee PL et al (2015) Structural covariance networks of striatum subdivision in patients with Parkinson's disease. Hum Brain Mapp 36:1567–1584
Alexander-Bloch A, Giedd JN, Bullmore E (2013) Imaging structural co-variance between human brain regions. Nat Rev Neurosci 14:322–336
Pagani M, Bifone A, Gozzi A (2016) Structural covariance networks in the mouse brain. NeuroImage 129:55–63
Paviour DC, Price SL, Stevens JM, Lees AJ, Fox NC (2005) Quantitative MRI measurement of superior cerebellar peduncle in progressive supranuclear palsy. Neurology 64:675–679
Marquand AF, Filippone M, Ashburner J et al (2013) Automated, high accuracy classification of Parkinsonian disorders: a pattern recognition approach. PLoS One 8:e69237
Lin WC, Chou KH, Lee PL et al (2017) Parkinson's disease: diagnostic utility of volumetric imaging. Neuroradiology 59:367–377
Fornito A, Zalesky A, Breakspear M (2015) The connectomics of brain disorders. Nat Rev Neurosci 16:159–172
Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD (2009) Neurodegenerative diseases target large-scale human brain networks. Neuron 62:42–52
Willette AA, Calhoun VD, Egan JM, Kapogiannis D, Alzheimers Disease Neuroimaging Initiative (2014) Prognostic classification of mild cognitive impairment and Alzheimer's disease: MRI independent component analysis. Psychiatry Res 224:81–88
Hafkemeijer A, Moller C, Dopper EG et al (2016) Differences in structural covariance brain networks between behavioral variant frontotemporal dementia and Alzheimer's disease. Hum Brain Mapp 37:978–988
Zeighami Y, Ulla M, Iturria-Medina Y et al (2015) Network structure of brain atrophy in de novo Parkinson's disease. elife. https://doi.org/10.7554/eLife.08440
Rektorova I, Biundo R, Marecek R, Weis L, Aarsland D, Antonini A (2014) Grey matter changes in cognitively impaired Parkinson's disease patients. PLoS One 9:e85595
Li X, Xing Y, Schwarz ST, Auer DP (2017) Limbic grey matter changes in early Parkinson's disease. Hum Brain Mapp. https://doi.org/10.1002/hbm.23610
Postuma RB, Berg D, Stern M et al (2015) MDS clinical diagnostic criteria for Parkinson's disease. Mov Disord 30:1591–1601
Gasser T, Bressman S, Durr A, Higgins J, Klockgether T, Myers RH (2003) State of the art review: molecular diagnosis of inherited movement disorders. Movement Disorders Society task force on molecular diagnosis. Mov Disord 18:3–18
Goetz CG, Poewe W, Rascol O et al (2004) Movement Disorder Society Task Force report on the Hoehn and Yahr staging scale: status and recommendations. Mov Disord 19:1020–1028
Schwab RS, Engeland A (1969) Projection technique for evaluating surgery in Parkinson's disease. Livingstone, Edinburgh
Ashburner J, Friston KJ (2000) Voxel-based morphometry–the methods. NeuroImage 11:805–821
Yang FC, Chou KH, Fuh JL et al (2013) Altered gray matter volume in the frontal pain modulation network in patients with cluster headache. Pain 154:801–807
Douaud G, Groves AR, Tamnes CK et al (2014) A common brain network links development, aging, and vulnerability to disease. Proc Natl Acad Sci U S A 111:17648–17653
Segall JM, Allen EA, Jung RE et al (2012) Correspondence between structure and function in the human brain at rest. Front Neuroinform 6:10
Smith SM, Fox PT, Miller KL et al (2009) Correspondence of the brain's functional architecture during activation and rest. Proc Natl Acad Sci U S A 106:13040–13045
Filippini N, MacIntosh BJ, Hough MG et al (2009) Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proc Natl Acad Sci U S A 106:7209–7214
Perkins NJ, Schisterman EF (2006) The inconsistency of "optimal" cutpoints obtained using two criteria based on the receiver operating characteristic curve. Am J Epidemiol 163:670–675
van Stralen KJ, Stel VS, Reitsma JB, Dekker FW, Zoccali C, Jager KJ (2009) Diagnostic methods I: sensitivity, specificity, and other measures of accuracy. Kidney Int 75:1257–1263
Nagelkerke NJD (1991) A note on a general definition of the coefficient of determination. Biometrika 78:691–692
Ortiz A, Munilla J, Alvarez-Illan I, Gorriz JM, Ramirez J, Alzheimer's Disease Neuroimaging Initiative (2015) Exploratory graphical models of functional and structural connectivity patterns for Alzheimer's Disease diagnosis. Front Comput Neurosci 9:132
Ng B, Varoquaux G, Poline JB, Thirion B, Greicius MD, Poston KL (2017) Distinct alterations in Parkinson's medication-state and disease-state connectivity. Neuroimage Clin 16:575–585
Zhang D, Liu X, Chen J, Liu B (2014) Distinguishing patients with Parkinson's disease subtypes from normal controls based on functional network regional efficiencies. PLoS One 9:e115131
Wang JJ, Lin WY, Lu CS et al (2011) Parkinson disease: diagnostic utility of diffusion kurtosis imaging. Radiology 261:210–217
Mahlknecht P, Krismer F, Poewe W, Seppi K (2017) Meta-analysis of dorsolateral nigral hyperintensity on magnetic resonance imaging as a marker for Parkinson's disease. Mov Disord 32:619–623
Szewczyk-Krolikowski K, Menke RA, Rolinski M et al (2014) Functional connectivity in the basal ganglia network differentiates PD patients from controls. Neurology 83:208–214
Wu T, Hallett M (2013) The cerebellum in Parkinson's disease. Brain 136:696–709
Lenka A, Naduthota RM, Jha M et al (2016) Freezing of gait in Parkinson's disease is associated with altered functional brain connectivity. Parkinsonism Relat Disord 24:100–106
Leh SE, Petrides M, Strafella AP (2010) The neural circuitry of executive functions in healthy subjects and Parkinson's disease. Neuropsychopharmacology 35:70–85
Carlesimo GA, Piras F, Assogna F, Pontieri FE, Caltagirone C, Spalletta G (2012) Hippocampal abnormalities and memory deficits in Parkinson disease: a multimodal imaging study. Neurology 78:1939–1945
Cardoso EF, Fregni F, Maia FM et al (2010) Abnormal visual activation in Parkinson's disease patients. Mov Disord 25:1590–1596
Luppino G, Matelli M, Camarda R, Rizzolatti G (1993) Corticocortical connections of area F3 (SMA-proper) and area F6 (pre-SMA) in the macaque monkey. J Comp Neurol 338:114–140
Cerasa A, Pugliese P, Messina D et al (2012) Prefrontal alterations in Parkinson's disease with levodopa-induced dyskinesia during fMRI motor task. Mov Disord 27:364–371
Sawada M, Imamura K, Nagatsu T (2006) Role of cytokines in inflammatory process in Parkinson's disease. J Neural Transm Suppl: 373–381
Monchi O, Petrides M, Mejia-Constain B, Strafella AP (2007) Cortical activity in Parkinson's disease during executive processing depends on striatal involvement. Brain 130:233–244
Funding
This work was supported by funds from the National Science Council (MOST 103-2314-B-182A-010 -MY3 to W-C Lin, MOST 104-2221-E-010-013- to C-P Lin, and MOST 104-2314-B-182A-053 to H-L Chen) and Chang Gung Memorial Hospital (CMRPG891511 and CMRPG8B0831 to W-C Lin, and CMRPG8E0621 to M-H Chen). The authors would like to thank the patients affected by PD and their families for their involvement and altruism. The authors declare no competing financial interests.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Wei-Che Lin in Kaohsiung Chang Gung Memorial Hospital.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
We declare that all human and animal studies have been approved by the Institutional Review Board of Chang Gung Memorial Hospital and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. We declare that all patients gave informed consent prior to inclusion in this study.
Methodology
• prospective
• diagnostic or prognostic study
• performed at one institution
Electronic supplementary material
ESM 1
(DOCX 270 kb)
Rights and permissions
About this article
Cite this article
Lee, PL., Chou, KH., Lu, CH. et al. Extraction of large-scale structural covariance networks from grey matter volume for Parkinson’s disease classification. Eur Radiol 28, 3296–3305 (2018). https://doi.org/10.1007/s00330-018-5342-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-018-5342-1