Abstract
Objectives
To assess the role of preoperative multiparametric MRI (mpMRI) of the prostate in the prediction of nodal metastases in patients treated with radical prostatectomy (RP) and extended pelvic lymph node dissection (ePLND).
Methods
We retrospectively analyzed 101 patients who underwent both preoperative mpMRI of the prostate and RP with ePLND at our institution. For each patient, complete preoperative clinical data and tumour characteristics at mpMRI were recorded. Final histopathologic stage was considered the standard of reference. Univariate and multivariate logistic regression analyses were performed.
Results
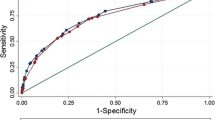
Nodal metastases were found in 23/101 (22.8%) patients. At univariate analyses, all clinical and radiological parameters were significantly associated to nodal invasion (all p<0.03); tumour volume at MRI (mrV), tumour ADC and tumour T-stage at MRI (mrT) were the most accurate predictors (AUC = 0.93, 0.86 and 0.84, respectively). A multivariate model including PSA levels, primary Gleason grade, mrT and mrV showed high predictive accuracy (AUC = 0.956). Observed prevalence of nodal metastases was very low among tumours with mrT2 stage and mrV<1cc (1.8%).
Conclusion
Preoperative mpMRI of the prostate can predict nodal metastases in prostate cancer patients, potentially allowing a better selection of candidates to ePLND.
Key points
• Multiparametric-MRI of the prostate can predict nodal metastases in prostate cancer
• Tumour volume and stage at MRI are the most accurate predictors
• Prevalence of nodal metastases is low for T2-stage and <1cc tumours
• Preoperative mpMRI may allow a better selection of candidates to lymphadenectomy

Similar content being viewed by others
Abbreviations
- PCa:
-
Prostate Cancer
- mpMRI:
-
Multi-parametric MRI
- RP:
-
Radical Prostatectomy
- ePLND:
-
Extended Pelvic Lymph Node Dissection
- LN:
-
Lymph Node
- N-staging:
-
Lymph Node Staging
- mrT-stage:
-
T-stage at MRI
- mrV:
-
Tumour Volume at MRI
References
Schumacher MC, Burkhard FC, Thalmann GN et al (2008) Good Outcome for Patients with Few Lymph Node Metastases After Radical Retropubic Prostatectomy. Eur Urol 54:344–352. https://doi.org/10.1016/j.eururo.2008.05.023
Mottet N, Bellmunt J, Bolla M et al (2017) EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur Urol 71:618–629. https://doi.org/10.1016/j.eururo.2016.08.003
Hövels AM, Heesakkers RAM, Adang EM et al (2008) The diagnostic accuracy of CT and MRI in the staging of pelvic lymph nodes in patients with prostate cancer: a meta-analysis. Clin Radiol 63:387–395. https://doi.org/10.1016/j.crad.2007.05.022
Briganti A, Abdollah F, Nini A et al (2012) Performance characteristics of computed tomography in detecting lymph node metastases in contemporary patients with prostate cancer treated with extended pelvic lymph node dissection. Eur Urol 61:1132–1138. https://doi.org/10.1016/j.eururo.2011.11.008
Thoeny HC, Froehlich JM, Triantafyllou M et al (2014) Metastases in Normal-sized Pelvic Lymph Nodes: Detection with Diffusion-weighted MR Imaging. Radiology 273:125–135. https://doi.org/10.1148/radiol.14132921
Van den Bergh L, Lerut E, Haustermans K et al (2015) Final analysis of a prospective trial on functional imaging for nodal staging in patients with prostate cancer at high risk for lymph node involvement. Urol Oncol 33:109.e23-109.e31. https://doi.org/10.1016/j.urolonc.2014.11.008
Harisinghani MG, Barentsz J, Hahn PF et al (2003) Noninvasive Detection of Clinically Occult Lymph-Node Metastases in Prostate Cancer. N Engl J Med 348:2491–2499. https://doi.org/10.1056/NEJMoa022749
Evangelista L, Guttilla A, Zattoni F et al (2013) Utility of choline positron emission tomography/computed tomography for lymph node involvement identification in intermediate- to high-risk prostate cancer: A systematic literature review and meta-analysis. Eur Urol 63:1040–1048. https://doi.org/10.1016/j.eururo.2012.09.039
Budaus L, Leyh-Bannurah SR, Salomon G et al (2016) Initial Experience of (68)Ga-PSMA PET/CT Imaging in High-risk Prostate Cancer Patients Prior to Radical Prostatectomy. Eur Urol 69:393–396. https://doi.org/10.1016/j.eururo.2015.06.010
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines).
Ploussard G, Briganti A, de la Taille A et al (2014) Pelvic lymph node dissection during robot-assisted radical prostatectomy: efficacy, limitations, and complications-a systematic review of the literature. Eur Urol 65:7–16. https://doi.org/10.1016/j.eururo.2013.03.057
Roach M, Marquez C, Yuo HS et al (1994) Predicting the risk of lymph node involvement using the pre-treatment prostate specific antigen and Gleason score in men with clinically localized prostate cancer. Int J Radiat Oncol Biol Phys 28:33–37. https://doi.org/10.1016/0360-3016(94)90138-4
Briganti A, Larcher A, Abdollah F et al (2012) Updated nomogram predicting lymph node invasion in patients with prostate cancer undergoing extended pelvic lymph node dissection: The essential importance of percentage of positive cores. Eur Urol 61:480–487. https://doi.org/10.1016/j.eururo.2011.10.044
Memorial Sloan Kettering Cancer Center Dynamic Prostate Cancer Nomogram: Coefficients. https://www.mskcc.org/nomograms/prostate/pre-op/coefficients.
Gandaglia G, Fossati N, Zaffuto E et al (2017) Development and Internal Validation of a Novel Model to Identify the Candidates for Extended Pelvic Lymph Node Dissection in Prostate Cancer. Eur Urol Article in press. https://doi.org/10.1016/j.eururo.2017.03.049
Barentsz JO, Richenberg J, Clements R et al (2012) ESUR prostate MR guidelines 2012. Eur Radiol 22:746–757. https://doi.org/10.1007/s00330-011-2377-y
Weinreb JC, Barentsz JO, Choyke PL et al (2016) PI-RADS Prostate Imaging - Reporting and Data System: 2015, Version 2. Eur Urol 69:16–40. https://doi.org/10.1016/j.eururo.2015.08.052
Jager GJ, Barentsz JO, Oosterhof GO et al (1996) Pelvic adenopathy in prostatic and urinary bladder carcinoma: MR imaging with a three-dimensional T1-weighted magnetization-prepared-rapid gradient- echo sequence. Am J Roentgenol 167:1503–1507
Burnham KP, Anderson DR (2002) Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach (2nd ed). Ecol Modell. https://doi.org/10.1016/j.ecolmodel.2003.11.004
Burnham KP, Anderson DR (2004) Multimodel Inference\rUnderstanding AIC and BIC in Model Selection. Sociol Methods Res 33:261–304
Wang L, Mullerad M, Chen H-N et al (2004) Prostate cancer: incremental value of endorectal MR imaging findings for prediction of extracapsular extension. Radiology 232:133–139. https://doi.org/10.1148/radiol.2321031086
Abdollah F, Suardi N, Gallina A et al (2013) Extended pelvic lymph node dissection in prostate cancer: A 20-year audit in a single center. Ann Oncol 24:1459–1466. https://doi.org/10.1093/annonc/mdt120
Stamey TA, Freiha FS, McNeal JE et al (1993) Localized prostate cancer. Relationship of tumor volume to clinical significance for treatment of prostate cancer. Cancer 71:933–938. https://doi.org/10.1002/1097-0142(19930201)71:3+<933::AID-CNCR2820711408>3.0.CO;2-L
McNeal JE, Villers AA, Redwine EA et al (1990) Histologic differentiation, cancer volume, and pelvic lymph node metastasis in adenocarcinoma of the prostate. Cancer 66:1225–1233. https://doi.org/10.1002/1097-0142(19900915)66:6<1225::AID-CNCR2820660624>3.0.CO;2-X
Knoedler JJ, Karnes RJ, Thompson RH et al (2014) The association of tumor volume with mortality following radical prostatectomy. Prostate Cancer Prostatic Dis 17:144–148. https://doi.org/10.1038/pcan.2013.61
Noguchi M, Stamey TA, McNeal JE, Yemoto CM (2001) Relationship between systematic biopsies and histological features of 222 radical prostatectomy specimens: lack of prediction of tumor significance for men with nonpalpable prostate cancer. J Urol 166:104–110
Humphrey PA, Jackbaty BA, Keetch D (1995) Relationship between Serum Prostate Specific Antigen, Needle Biopsy Findings, and Histopathologic Features of Prostatic Carcinoma in Radical Prostatectomy Tissues. Cancer 75:1842–1849
Coakley FV, Kurhanewicz J, Lu Y et al (2002) Prostate Cancer Tumor Volume: Measurement with Endorectal MR and MR Spectroscopic Imaging. Radiology 223:91–97. https://doi.org/10.1148/radiol.2231010575
Turkbey B, Mani H, Aras O et al (2012) Correlation of Magnetic Resonance Imaging Tumor Volume with Histopathology. J Urol 188:1157–1163. https://doi.org/10.1016/j.juro.2012.06.011
Harvey H, Orton MR, Morgan VA et al (2017) Volumetry of the dominant intraprostatic tumour lesion: Intersequence and interobserver differences on multiparametric MRI. Br J Radiol 90:20160416. https://doi.org/10.1259/bjr.20160416
Wang L, Hricak H, Kattan MW et al (2006) Combined endorectal and phased-array MRI in the prediction of pelvic lymph node metastasis in prostate cancer. Am J Roentgenol 186:743–748. https://doi.org/10.2214/AJR.04.1682
Park SY, Oh YT, Jung DC et al (2015) Prediction of Micrometastasis (< 1 cm) to Pelvic Lymph Nodes in Prostate Cancer: Role of Preoperative MRI. Am J Roentgenol 205:W328–W334. https://doi.org/10.2214/AJR.14.14138
Park SY, Shin S-J, Jung DC et al (2017) PI-RADS version 2: Preoperative role in the detection of normal-sized pelvic lymph node metastasis in prostate cancer. Eur J Radiol 91:22–28. https://doi.org/10.1016/j.ejrad.2017.03.009
Abdollah F, Sun M, Thuret R et al (2010) Decreasing rate and extent of lymph node staging in patients undergoing radical prostatectomy may undermine the rate of diagnosis of lymph node metastases in prostate cancer. Eur Urol 58:882–892. https://doi.org/10.1016/j.eururo.2010.09.029
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Francesco De Cobelli, M.D.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
Alessandro Ambrosi Ph.D. kindly provided statistical advice for this manuscript, and is one of the authors.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• observational
• performed at one institution
Rights and permissions
About this article
Cite this article
Brembilla, G., Dell’Oglio, P., Stabile, A. et al. Preoperative multiparametric MRI of the prostate for the prediction of lymph node metastases in prostate cancer patients treated with extended pelvic lymph node dissection. Eur Radiol 28, 1969–1976 (2018). https://doi.org/10.1007/s00330-017-5229-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-017-5229-6