Abstract
Objectives
To investigate the influence of region-of-interest (ROI) placement and different apparent diffusion coefficient (ADC) parameters on ADC values, diagnostic performance, reproducibility and measurement time in breast tumours.
Methods
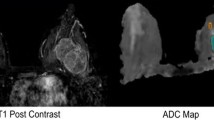
In this IRB-approved, retrospective study, 149 histopathologically proven breast tumours (109 malignant, 40 benign) in 147 women (mean age 53.2) were investigated. Three radiologists independently measured minimum, mean and maximum ADC, each using three ROI placement approaches:1 – small 2D-ROI, 2 – large 2D-ROI and 3 – 3D-ROI covering the whole lesion. One reader performed all measurements twice. Median ADC values, diagnostic performance, reproducibility, and measurement time were calculated and compared between all combinations of ROI placement approaches and ADC parameters.
Results
Median ADC values differed significantly between the ROI placement approaches (p < .001). Minimum ADC showed the best diagnostic performance (AUC .928–.956), followed by mean ADC obtained from 2D ROIs (.926–.94). Minimum and mean ADC showed high intra- (ICC .85–.94) and inter-reader reproducibility (ICC .74–.94). Median measurement time was significantly shorter for the 2D ROIs (p < .001).
Conclusions
ROI placement significantly influences ADC values measured in breast tumours. Minimum and mean ADC acquired from 2D-ROIs are useful for the differentiation of benign and malignant breast lesions, and are highly reproducible, with rapid measurement.
Key Points
• Region of interest placement significantly influences apparent diffusion coefficient of breast tumours.
• Minimum and mean apparent diffusion coefficient perform best and are reproducible.
• 2D regions of interest perform best and provide rapid measurement times.





Similar content being viewed by others
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- AUC:
-
Area under the curve
- CE:
-
Contrast enhanced
- CI:
-
Confidence interval
- DCIS:
-
Ductal carcinoma in situ
- DWI:
-
Diffusion weighted imaging
- EPI:
-
Echo planar imaging
- ICC:
-
Intra-class correlation
- IDC:
-
Invasive ductal carcinoma
- ILC:
-
Invasive lobular carcinoma
- IPC:
-
Intraductal papillary carcinoma
- MPR:
-
Multiplanar reconstruction
- MRI:
-
Magnetic resonance imaging
- ROC:
-
Receiver operating characteristics
- ROI:
-
Region of interest
References
Bogner W, Pinker K, Zaric O et al (2015) Bilateral diffusion-weighted MR imaging of breast tumors with submillimeter resolution using readout-segmented echo-planar imaging at 7 T. Radiology 274:74–84
Bickel H, Pinker-Domenig K, Bogner W et al (2015) Quantitative apparent diffusion coefficient as a noninvasive imaging biomarker for the differentiation of invasive breast cancer and ductal carcinoma in situ. Invest Radiol 50:95–100
Woodhams R, Matsunaga K, Kan S et al (2005) ADC mapping of benign and malignant breast tumors. Magn Reson Med Sci 4:35–42
Pinker K, Bickel H, Helbich TH et al (2013) Combined contrast-enhanced magnetic resonance and diffusion-weighted imaging reading adapted to the "Breast Imaging Reporting and Data System" for multiparametric 3-T imaging of breast lesions. Eur Radiol 23:1791–1802
Spick C, Pinker-Domenig K, Rudas M, Helbich TH, Baltzer PA (2014) MRI-only lesions: application of diffusion-weighted imaging obviates unnecessary MR-guided breast biopsies. Eur Radiol 24:1204–1210
Sun K, Chai W, Fu C et al (2015) Diffusion-weighted imaging-guided MR spectroscopy in breast lesions using readout-segmented echo-planar imaging. Eur Radiol. doi:10.1007/s00330-015-4000-0
Woodhams R, Matsunaga K, Iwabuchi K et al (2005) Diffusion-weighted imaging of malignant breast tumors: the usefulness of apparent diffusion coefficient (ADC) value and ADC map for the detection of malignant breast tumors and evaluation of cancer extension. J Comput Assist Tomogr 29:644–649
Baltzer PA, Schafer A, Dietzel M et al (2011) Diffusion tensor magnetic resonance imaging of the breast: a pilot study. Eur Radiol 21:1–10
Dorrius MD, Dijkstra H, Oudkerk M, Sijens PE (2014) Effect of b value and pre-admission of contrast on diagnostic accuracy of 1.5-T breast DWI: a systematic review and meta-analysis. Eur Radiol 24:2835–2847
Lourenco AP, Donegan L, Khalil H, Mainiero MB (2014) Improving outcomes of screening breast MRI with practice evolution: initial clinical experience with 3T compared to 1.5T. J Magn Reson Imaging 39:535–539
Gruber S, Minarikova L, Pinker K et al (2016) Diffusion-weighted imaging of breast tumours at 3 Tesla and 7 Tesla: a comparison. Eur Radiol 26:1466–1473
Bogner W, Gruber S, Pinker K et al (2009) Diffusion-weighted MR for differentiation of breast lesions at 3.0 T: how does selection of diffusion protocols affect diagnosis? Radiology 253:341–351
Barentsz MW, Taviani V, Chang JM et al (2015) Assessment of tumor morphology on diffusion-weighted (DWI) breast MRI: diagnostic value of reduced field of view DWI. J Magn Reson Imaging 42:1656–1665
Clauser P, Marcon M, Maieron M, Zuiani C, Bazzocchi M, Baltzer PA (2015) Is there a systematic bias of apparent diffusion coefficient (ADC) measurements of the breast if measured on different workstations? an inter- and intra-reader agreement study. Eur Radiol. doi:10.1007/s00330-015-4051-2
Lambregts DM, Beets GL, Maas M et al (2011) Tumour ADC measurements in rectal cancer: effect of ROI methods on ADC values and interobserver variability. Eur Radiol 21:2567–2574
Hatakenaka M, Soeda H, Yabuuchi H et al (2008) Apparent diffusion coefficients of breast tumors: clinical application. Magn Reson Med Sci 7:23–29
Choi SY, Chang YW, Park HJ, Kim HJ, Hong SS, Seo DY (2012) Correlation of the apparent diffusion coefficiency values on diffusion-weighted imaging with prognostic factors for breast cancer. Br J Radiol 85:474–479
Iacconi C, Giannelli M, Marini C et al (2010) The role of mean diffusivity (MD) as a predictive index of the response to chemotherapy in locally advanced breast cancer: a preliminary study. Eur Radiol 20:303–308
Mori N, Ota H, Mugikura S et al (2013) Detection of invasive components in cases of breast ductal carcinoma in situ on biopsy by using apparent diffusion coefficient MR parameters. Eur Radiol 23:2705–2712
Hirano M, Satake H, Ishigaki S, Ikeda M, Kawai H, Naganawa S (2012) Diffusion-weighted imaging of breast masses: comparison of diagnostic performance using various apparent diffusion coefficient parameters. AJR Am J Roentgenol 198:717–722
Partridge SC, Rahbar H, Murthy R et al (2011) Improved diagnostic accuracy of breast MRI through combined apparent diffusion coefficients and dynamic contrast-enhanced kinetics. Magn Reson Med 65:1759–1767
Pinker K, Bogner W, Baltzer P et al (2014) Improved diagnostic accuracy with multiparametric magnetic resonance imaging of the breast using dynamic contrast-enhanced magnetic resonance imaging, diffusion-weighted imaging, and 3-dimensional proton magnetic resonance spectroscopic imaging. Invest Radiol 49:421–430
Sardanelli F, Boetes C, Borisch B et al (2010) Magnetic resonance imaging of the breast: recommendations from the EUSOMA working group. Eur J Cancer 46:1296–1316
Rosset A, Spadola L, Pysher L, Ratib O (2006) Informatics in radiology (infoRAD): navigating the fifth dimension: innovative interface for multidimensional multimodality image navigation. Radiographics 26:299–308
Perry N, Broeders M, de Wolf C, Tornberg S, Holland R, von Karsa L (2008) European guidelines for quality assurance in breast cancer screening and diagnosis. Fourth edition--summary document. Ann Oncol 19:614–622
Vergara IA, Norambuena T, Ferrada E, Slater AW, Melo F (2008) StAR: a simple tool for the statistical comparison of ROC curves. BMC Bioinform 9:265
Arponent O, Sudah M, Masarwah A (2015) Diffusion-weighted imaging in 3.0 Tesla breast MRI: diagnostic performance and tumor characterization using small subregions vs. whole tumor regions of interest. PLoS One 10:e0138702
Nogueira L, Brandao S, Matos E et al (2015) Region of interest demarcation for quantification of the apparent diffusion coefficient in breast lesions and its interobserver variability. Diagn Interv Radiol 21:123–127
Min Q, Shao K, Zhai L et al (2015) Differential diagnosis of benign and malignant breast masses using diffusion-weighted magnetic resonance imaging. World J Surg Oncol 13:32
Guo Y, Cai YQ, Cai ZL et al (2002) Differentiation of clinically benign and malignant breast lesions using diffusion-weighted imaging. J Magn Reson Imaging 16:172–178
Kul S, Eyuboglu I, Cansu A, Alhan E (2014) Diagnostic efficacy of the diffusion weighted imaging in the characterization of different types of breast lesions. J Magn Reson Imaging 40:1158–1164
Partridge SC, Mullins CD, Kurland BF et al (2010) Apparent diffusion coefficient values for discriminating benign and malignant breast MRI lesions: effects of lesion type and size. AJR Am J Roentgenol 194:1664–1673
Iima M, Le Bihan D, Okumura R et al (2011) Apparent diffusion coefficient as an MR imaging biomarker of low-risk ductal carcinoma in situ: a pilot study. Radiology 260:364–372
(2010) European Society of Radiology. white paper on imaging biomarkers. Insights Imag 1:42–45
Bogner W, Pinker-Domenig K, Bickel H et al (2012) Readout-segmented echo-planar imaging improves the diagnostic performance of diffusion-weighted MR breast examinations at 3.0 T. Radiology 263:64–76
Dijkstra H, Dorrius MD, Wielema M et al (2016) Semi-automated quantitative intravoxel incoherent motion analysis and its implementation in breast diffusion-weighted imaging. J Magn Reson Imaging 43:1122–1131
Giannotti E, Waugh S, Priba L, Davis Z, Crowe E, Vinnicombe S (2015) Assessment and quantification of sources of variability in breast apparent diffusion coefficient (ADC) measurements at diffusion weighted imaging. Eur J Radiol 84:1729–1736
Pereira FP, Martins G, Figueiredo E et al (2009) Assessment of breast lesions with diffusion-weighted MRI: comparing the use of different b values. AJR Am J Roentgenol 193:1030–1035
Baltzer PAT, Renz DM, Herrmann KH et al (2009) Diffusion-weighted imaging (DWI) in MR mammography (MRM): clinical comparison of echo planar imaging (EPI) and half-Fourier single-shot turbo spin echo (HASTE) diffusion techniques. Eur Radiol 19:1612–1620
Lo GG, Ai V, Chan JK et al (2009) Diffusion-weighted magnetic resonance imaging of breast lesions: first experiences at 3 T. J Comput Assist Tomogr 33:63–69
Kamitani T, Matsuo Y, Yabuuchi H et al (2013) Correlations between apparent diffusion coefficient values and prognostic factors of breast cancer. Magn Reson Med Sci 12:193–199
Acknowledgments
The scientific guarantor of this publication is Thomas Helbich. The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article. This study has received funding by projects no. 13652 funded by Austrian National Bank ‘Jubilaeumsfond’ and no. 10029 funded by the Medical-Scientific Funds of the Mayor of Vienna.
One of the authors (Pascal Baltzer) has significant statistical expertise. Institutional Review Board approval was obtained. Written informed consent was obtained from all subjects (patients) in this study. Approval from the institutional animal care committee was not required because no animals were involved in this study. Some study subjects or cohorts have been previously reported in:
Pinker K, Bickel H, Helbich TH, et al. Combined contrast-enhanced magnetic resonance and diffusion-weighted imaging reading adapted to the "Breast Imaging Reporting and Data System" for multiparametric 3-T imaging of breast lesions. Eur Radiol. 2013;23(7):1791-802. (n = 85)
Pinker K, Bogner W, Baltzer P, et al. Improved diagnostic accuracy with multiparametric magnetic resonance imaging of the breast using dynamic contrast-enhanced magnetic resonance imaging, diffusion-weighted imaging and 3-dimensional proton magnetic resonance spectroscopic imaging. Investigative radiology. 2014;49(6):421-30. (n = 52)
Pinker K, Bogner W, Baltzer P, et al. Improved differentiation of benign and malignant breast tumours with multiparametric 18fluorodeoxyglucose positron emission tomography magnetic resonance imaging: a feasibility study. Clinical cancer research : an official journal of the American Association for Cancer Research. 2014;20(13):3540-9. (n = 39)
Bickel H, Pinker-Domenig K, Bogner W, et al. Quantitative apparent diffusion coefficient as a noninvasive imaging biomarker for the differentiation of invasive breast cancer and ductal carcinoma in situ. Investigative radiology. 2015;50(2):95-100. (n = 83)
Methodology: retrospective, diagnostic or prognostic study, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Table 1
Results of the ROC analysis comparing the different measurement approaches. Note: Differences in the AUC are shown in the upper right triangle; the corresponding p-values are presented in the lower left triangle. Non-significant results are highlighted in gray. Abbreviations: ROC – receiver operating characteristics; AUC – area under the curve; Min – minimum; Max – maximum. (DOCX 74 kb)
Supplemental Table 2
Results of the ROC analysis comparing the different measurement approaches, divided by mass/non-mass enhancement. Note: Non-significant results are highlighted in gray. Abbreviations: ROC – receiver operating characteristics; AUC – area under the curve; SD – standard deviation; Min – minimum; Max – maximum. (DOCX 72 kb)
Supplemental Table 3
Results of the ROC analysis comparing the different measurement approaches in mass lesions only. Note: Differences in the AUC are shown in the upper right triangle; the corresponding p-values are presented in the lower left triangle. Non-significant results are highlighted in gray. Abbreviations: ROC – receiver operating characteristics; AUC – area under the curve; Min – minimum; Max – maximum. (DOCX 87 kb)
Supplemental Table 4
Results of the ROC analysis comparing the different measurement approaches in non-mass lesions only. Note: Differences in the AUC are shown in the upper right triangle; the corresponding p-values are presented in the lower left triangle. Non-significant results are highlighted in gray. Abbreviations: ROC – receiver operating characteristics; AUC – area under the curve; Min – minimum; Max – maximum (DOCX 84 kb)
Rights and permissions
About this article
Cite this article
Bickel, H., Pinker, K., Polanec, S. et al. Diffusion-weighted imaging of breast lesions: Region-of-interest placement and different ADC parameters influence apparent diffusion coefficient values. Eur Radiol 27, 1883–1892 (2017). https://doi.org/10.1007/s00330-016-4564-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-016-4564-3