Abstract
Objectives
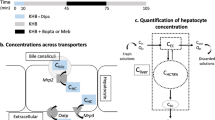
Changes in the expression of hepatocyte membrane transporters in advanced fibrosis decrease the hepatic transport function of organic anions. The aim of our study was to assess if these changes can be evaluated with pharmacokinetic analysis of the hepatobiliary transport of the MR contrast agent gadoxetate.
Methods
Dynamic gadoxetate-enhanced MRI was performed in 17 rats with advanced fibrosis and 8 normal rats. After deconvolution, hepatocyte three-compartmental analysis was performed to calculate the hepatocyte influx, biliary efflux and sinusoidal backflux rates. The expression of Oatp1a1, Mrp2 and Mrp3 organic anion membrane transporters was assessed with reverse transcription polymerase chain reaction.
Results
In the rats with advanced fibrosis, the influx and efflux rates of gadoxetate decreased and the backflux rate increased significantly (p = 0.003, 0.041 and 0.010, respectively). Significant correlations were found between influx and Oatp1a1 expression (r = 0.78, p < 0.001), biliary efflux and Mrp2 (r = 0.50, p = 0.016) and sinusoidal backflux and Mrp3 (r = 0.61, p = 0.002).
Conclusion
These results show that changes in the bidirectional organic anion hepatocyte transport function in rats with advanced liver fibrosis can be assessed with compartmental analysis of gadoxetate-enhanced MRI.
Key Points
• Expression of hepatocyte transporters is modified in rats with advanced liver fibrosis.
• Kinetic parameters at gadoxetate-enhanced MRI are correlated with hepatocyte transporter expression.
• Hepatocyte transport function can be assessed with compartmental analysis of gadoxetate-enhanced MRI.
• Compartmental analysis of gadoxetate-enhanced MRI might provide biomarkers in advanced liver fibrosis.





Similar content being viewed by others
References
International Transporter Consortium, Giacomini KM, Huang SM et al (2010) Membrane transporters in drugdevelopment. Nat Rev Drug Discov 9:215–236
Stieger B, Heger M, de Graaf W, Paumgartner G, van Gulik T (2012) The emerging role of transport systems in liver function tests. Eur J Pharmacol 675:1–5
Roth M, Obaidat A, Hagenbuch B (2012) OATPs, OATs and OCTs: the organic anion and cation transporters of the SLCO and SLC22A gene superfamilies. Br J Pharmacol 165:1260–1287
Gu X, Manautou JE (2012) Regulation of hepatic ABCC transporters by xenobiotics and in disease states. Drug Metab Rev 42:482–538
Van Beers BE, Pastor CM, Hussain HK (2012) Primovist, Eovist: what to expect? J Hepatol 57:421–429
Jia J, Puls D, Oswald S et al (2014) Characterization of the intestinal and hepatic uptake/efflux transport of the magnetic resonance imaging contrast agent gadolinium-ethoxylbenzyl-diethylenetriamine-pentaacetic acid. Investig Radiol 49:78–86
Gambhir SS, Hawkins RA, Huang SC, Hall TR, Busuttil RW, Phelps ME (1989) Tracer kinetic modeling approaches for the quantification of hepatic function with technetium-99m DISIDA and scintigraphy. J Nucl Med 30:1507–1518
Araikum S, Mdaka T, Esser JD, Zuckerman M (1996) Hepatobiliary kinetics of technetium-99m-IDA analogs: quantification by linear systems theory. J Nucl Med 37:1323–1330
Peters AM (1998) Fundamental of tracer kinetics for radiologists. Br J Radiol 71:1116–1129
Nilsson H, Nordell A, Vargas R, Douglas L, Jonas E, Blomqvist L (2009) Assessment of hepatic extraction fraction and input relative blood flow using dynamic hepatocyte-specific contrast-enhanced MRI. J Magn Reson Imaging 29:1323–1331
Sourbron S, Sommer WH, Reiser MF, Zech CJ (2012) Combined quantification of liver perfusion and function with dynamic gadoxetic acid-enhanced MR imaging. Radiology 263:874–883
Nilsson H, Blomqvist L, Douglas L, Nordell A, Jonas E (2010) Assessment of liver function in primary biliary cirrhosis using Gd-EOB-DTPA-enhanced liver MRI. HPB 12:567–576
Nilsson H, Blomqvist L, Douglas L et al (2013) Gd-EOB-DTPA-enhanced MRI for the assessment of liver function and volume in liver cirrhosis. Br J Radiol 86:20120653
Lagadec M, Doblas S, Giraudeau C et al (2015) Advanced fibrosis: correlation between pharmacokinetic parameters at dynamic gadoxetate-enhanced MR imaging and hepatocyte organic anion transporter expression in rat liver. Radiology 274:379–386
Haimerl M, Schlabeck M, Verloh N et al (2016) Volume-assisted estimation of liver function based on Gd-EOB-DTPA-enhanced MR relaxometry. Eur Radiol 26:1125–1133
Starkel P, Leclercq IA (2011) Animal models for the study of hepatic fibrosis. Best Pract Res Clin Gastroenterol 25:319–333
Rusinek H, Lee VS, Johnson G (2001) Optimal dose of Gd-DTPA in dynamic MR studies. Magn Reson Med 46:312–316
Braet F, Wisse E (2002) Structural and functional aspects of liver sinusoidal endothelial cell fenestrae: a review. Comp Hepatol 1:1–17
Van Beers BE, Materne R, Annet L et al (2003) Capillarization of the sinusoids in liver fibrosis: noninvasive assessment with contrast-enhanced MRI in the rabbit. Magn Reson Med 49:692–699
Veteläinen RL, Bennink RJ, de Bruin K, van Vliet A, van Gulik TM (2006) Hepatobiliary function assessed by 99mTc-mebrofenin cholescintigraphy in the evaluation of severity of steatosis in a rat model. Eur J Nucl Med Mol Imaging 33:1107–1114
Blouin A, Bolender RP, Weibel ER (1977) Distribution of organelles and membranes between hepatocytes and non hepatocytes in the rat liver parenchyma. A stereological study. J Cell Biol 72:441–455
Bedossa P (1994) Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. Hepatology 20:15–20
Peters AM, Myers MJ, Mohammadtaghi S, Mubashar M, Mathie RT (1998) Bidirectional transport of iminodiacetic organic anion analogues between plasma and hepatocyte. Eur J Nucl Med 25:766–773
Pfeifer ND, Hardwick RN, Brouwer KL (2014) Role of hepatic efflux transporters in regulating systemic and hepatocyte exposure to xenobiotics. Annu Rev Pharmacol Toxicol 54:509–535
Yoon JH, Lee JM, Paek M, Han JK, Choi BI (2016) Quantitative assessment of hepatic function: modified look-locker inversion recovery (MOLLI) sequence for T1 mapping on Gd-EOB-DTPA-enhanced liver MR imaging. Eur Radiol 26:1775–1782
Geisel D, Lüdemann L, Fröling V et al (2015) Imaging-based evaluation of liver function: comparison of 99mTc-mebrofenin hepatobiliary scintigraphy and Gd-EOB-DTPA-enhanced. MRI. Eur Radiol 25:1384–1391
Ba-Ssalamah A, Bastatai N, Wibmer A et al (2016) Hepatic gadoxetic acid uptake as a measure of liver disease: where are we? J Magn Reson Imaging, in press
Materne R, Van Beers BE, Smith AM et al (2000) Non-invasive quantification of liver perfusion with dynamic computed tomography and a dual-input one-compartmental model. Clin Sci 99:517–525
Kershaw LE, Buckley DL (2006) Precision in measurements of perfusion and microvascular permeability with T1-weighted dynamic contrast-enhanced MRI. Magn Reson Med 56:986–992
Sanz-Requena R, Prats-Montalbán JM, Martí-Bonmatí L (2015) Automatic individual arterial input functions calculated from PCA outperform manual and population-averaged approaches for the pharmacokinetic modeling of DCE-MR images. J Magn Reson Imaging 42:477–487
Heye T, Merkle EM, Reiner CS et al (2013) Reproducibility of dynamic contrast-enhanced MR imaging. Part II. Comparison of intra- and interobserver variability with manual region of interest placement versus semiautomatic lesion segmentation and histogram analysis. Radiology 266:812–821
Schuhmann-Giampieri G, Schmitt-Willich H, Press WR, Negishi C, Weinmann HJ, Speck U (1992) Preclinical evaluation of Gd-EOB-DTPA as a contrast agent in MR imaging of the hepatobiliary system. Radiology 183:59–64
Shen Y, Goerner FL, Snyder C et al (2015) T1 relaxivities of gadolinium-based magnetic resonance contrast agents in human whole blood at 1.5, 3, and 7 T. Investig Radiol 50:330–338
Rohrer M, Bauer H, Mintorovitch J, Requardt M, Weinmann HJ (2005) Comparison of magnetic properties of MRI contrast media solutions at different magnetic field strengths. Investig Radiol 40:715–724h
Acknowledgments
The authors thank Valérie Paradis (department of pathology, Beaujon University Hospital Paris Nord, Clichy, France) for performing the histopathological studies. The scientific guarantor of this publication is Bernard E. Van Beers. The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article. The authors state that this work has not received any funding. No complex statistical methods were necessary for this paper. Approval from the institutional animal care committee was obtained. Methodology: retrospective, experimental, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
By assuming linear tracer kinetics to describe uptake and elimination of gadoxetate in hepatocytes [12], kinetic indexes can be derived by the linear system theory such as:
The xi(t), j, i = 1…n with n the number of compartments, describe the time t evolution of gadoxetate in each compartment. Aji is the flow into and out of each compartment, ui(t) are the input control functions for each compartment and Bji the matrix describing the method of control application. By integrating Eq. 1.3 with xi(0) = 0 for all i as initial conditions:
Here, h(t) can be decomposed as the sum of the evolution of the gadotexate concentration into two compartments (hepatocytes and intra-hepatic bile ducts):
Rights and permissions
About this article
Cite this article
Giraudeau, C., Leporq, B., Doblas, S. et al. Gadoxetate-enhanced MR imaging and compartmental modelling to assess hepatocyte bidirectional transport function in rats with advanced liver fibrosis. Eur Radiol 27, 1804–1811 (2017). https://doi.org/10.1007/s00330-016-4536-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-016-4536-7