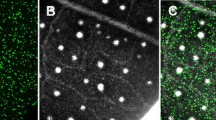
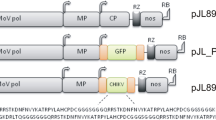
Abstract
Key message
We developed a non-packaged CMV system (NoPaCS) for CMV-agroinfection with a virus-inescapable transgenic plant platform, enabling rapid, high production of a large-sequence target protein.
Abstract
For rapidly producing high levels of a desirable protein, many plant virus vectors have been developed. However, there is always a concern that such recombinant viruses may escape into the environment. Especially for insect-transmissible viruses, certain measures must be taken. We here developed a new cucumber mosaic virus (CMV) RNA 3-based vector that is not transmitted by aphids because we deleted the coat protein (CP) gene responsible for aphid transmission and replaced it with a foreign gene. Transgenic Nicotiana benthamiana plants expressing CMV RNA 1 (CR1Tg) were found to be the most suitable platform for producing a recombinant protein using the CMV vector. By agroinfiltrating CR1Tg plants with the RNA 2 construct and the CMV vector harboring the green fluorescence protein (GFP) gene instead of the CP gene, we achieved a high yield of GFP (e.g., ~ 750 mg/kg FW) throughout the bacteria-infiltrated tissues at 2–3 days after infiltration. Furthermore, with this CMV-agroinfection system, a large gene such as the β-glucuronidase (GUS) gene can be expressed because the viral RNAs are not necessarily encapsidated for replication. The system is designated “non-packaged CMV system (NoPaCS)”.






Similar content being viewed by others
References
Cañizares MC, Liu L, Perrin Y, Tsakiris E, Lomonossoff GP (2006) A bipartite system for the constitutive and inducible expression of high levels of foreign proteins in plants. Plant Biotechnol J 4:183–193
Chen B, Francki RIB (1990) Cucumovirus transmission by the aphid Myzus persicae is determined solely by the viral coat protein. J Gen Virol 71:939–944
Fujiki M, Kaczmarczyk JF, Yusibov V, Rabindran S (2008) Development of a new cucumber mosaic virus-based plant expression vector with truncated 3a movement protein. Virology 381:136–142
Fukuzawa N, Ishihara T, Itchoda N, Tabayashi N, Kataoka C, Masuta C, Matsumura T (2011) Risk-managed production of bioactive recombinant proteins using a novel plant virus vector with a helper plant to complement viral systemic movement. Plant Biotechnol J 9:38–49
Fukuzawa N, Matsumura T, Masuta C (2018) Useful application of CMV. In: Garcia-Arenal F, Palukaitis P (eds) Cucumber mosaic virus. APS Press, St Paul (in press)
Giritch A, Marillonnet S, Engler C, van Eldik G, Botterman J, Klimyuk V, Gleba Y (2006) Rapid high-yield expression of full-size IgG antibodies in plants coinfected with noncompeting viral vectors. Proc Natl Acad Sci USA 103:14701–14706
Goto K, Kobori T, Kosaka Y, Natsuaki T, Masuta C (2007) Characterization of silencing suppressor 2b of cucumber mosaic virus based on examination of its small RNA-binding abilities. Plant Cell Physiol 48:1050–1060
Green BJ, Fujiki M, Mett V, Kaczmarczyk J, Shamloul M, Musiychuk K, Underkoffler S, Yusibov V, Mett V (2009) Transient protein expression in three Pisum sativum (green pea) varieties. Biotechnol J 4:230–237
Horsch RB, Fry JE, Hoffmann NL, Eichholtz D, Rogers SG, Fraley RT (1985) A simple and general method for transferring genes into plants. Science 227:1229–1231
Hwang MS, Lindenmuth BE, McDonald KA, Falk BW (2012) Bipartite and tripartite Cucumber mosaic virus-based vectors for producing the Acidothermus cellulolyticus endo-1,4-β-glucanase and other proteins in non-transgenic plants. BMC Biotechnol 12:66. https://doi.org/10.1186/1472-6750-12-66
Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6:3901–3907
Kim SH, Kalinina NO, Andreev I, Ryabov EV, Fitzgerald AG, Taliansky ME, Palukaitis P (2004) The C-terminal 33 amino acids of the cucumber mosaic virus 3a protein affect virus movement, RNA binding and inhibition of infection and translation. J Gen Virol 85:221–230
Marillonnet S, Thoeringer C, Kandzia R, Klimyuk V, Gleba Y (2005) Systemic Agrobacterium tumefaciens-mediated transfection of viral replicons for efficient transient expression in plants. Nat Biotechnol 23:718–723
Matsuo K, Hong JS, Tabayashi N, Ito A, Masuta C, Matsumura T (2007) Development of Cucumber mosaic virus as a vector modifiable for different host species to produce therapeutic proteins. Planta 225:277–286
Nagano H, Okuno T, Mise K, Furusawa I (1997) Deletion of the C-terminal 33 amino acids of cucumber mosaic virus movement protein enables a chimeric brome mosaic virus to move cell to cell. J Virol 71:2270–2276
Nagano H, Mise K, Furusawa I, Okuno T (2001) Conversion in the requirement of coat protein in cell-to-cell movement mediated by the cucumber mosaic virus movement protein. J Virol 75:8045–8053
Ng JC, Josefsson C, Clark AJ, Franz AW, Perry KL (2005) Virion stability and aphid vector transmissibility of Cucumber mosaic virus mutants. Virology 332:397–405
Nguyen L, Lucas WJ, Ding B, Zaitlin M (1996) Viral RNA trafficking is inhibited in replicase-mediated resistant transgenic tobacco plants. Proc Natl Acad Sci USA 93:12643–12647
Olinger GG Jr, Pettitt J, Kim D, Working C, Bohorov O, Bratcher B, Hiatt E, Hume SD, Johnson AK, Morton J, Pauly M, Whaley KJ, Lear CM, Biggins JE, Scully C, Hensley L, Zeitlin L (2012) Delayed treatment of Ebola virus infection with plant-derived monoclonal antibodies provides protection in rhesus macaques. Proc Natl Acad Sci USA 109:18030–18035
Palukaitis P, García-Arenal F (2003) Cucumoviruses. Adv Virus Res 62:241–323
Pettitt J, Zeitlin L, Kim DH, Working C, Johnson JC, Bohorov O, Bratcher B, Hiatt E, Hume SD, Johnson AK, Morton J, Pauly MH, Whaley KJ, Ingram MF, Zovanyi A, Heinrich M, Piper A, Zelko J, Olinger GG (2013) Therapeutic intervention of Ebola virus infection in rhesus macaques with the MB-003 monoclonal antibody cocktail. Sci Transl Med 5:199ra113
Qi Y, Zhong X, Itaya A, Ding B (2004) Dissecting RNA silencing in protoplasts uncovers novel effects of viral suppressors on the silencing pathway at the cellular level. Nucleic Acids Res 32:e179
Qiu X, Wong G, Fernando L, Audet J, Bello A, Strong J, Alimonti JB, Kobinger GP (2013) mAbs and Ad-vectored IFN-α therapy rescue Ebola-infected nonhuman primates when administered after the detection of viremia and symptoms. Sci Transl Med 5:207ra143
Qiu X, Wong G, Audet J, Bello A, Fernando L, Alimonti JB, Fausther-Bovendo H, Wei H, Aviles J, Hiatt E, Johnson A, Morton J, Swope K, Bohorov O, Bohrova N, Goodman C, Kim D, Pauly MH, Velasco J, Pettitte J, Olinger GG, Whaley K, Xu B, Strong JE, Zeitlin L, Kobinger GP (2014) Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp™. Nature 514:47–53
Suzuki M, Kuwata S, Kataoka J, Masuta C, Nitta N, Takanami Y (1991) Functional analysis of deletion mutants of cucumber mosaic virus RNA3 using an in vitro transcription system. Virology 183:106–113
Suzuki M, Masuta C, Takanami Y, Kuwata S (1996) Resistance against cucumber mosaic virus in plants expressing the viral replicon. FEBS Lett 379:26–30
Takeshita M, Koizumi E, Noguchi M, Sueda K, Shimura H, Ishikawa N, Matsuura H, Ohshima K, Natsuaki T, Kuwata S, Furuya N, Tsuchiya K, Masuta C (2012) Infection dynamics in viral spread and interference under the synergism between Cucumber mosaic virus and Turnip mosaic virus. Mol Plant Microbe Interact 25:18–27
Werner S, Breus O, Symonenko Y, Marillonnet S, Gleba Y (2011) High-level recombinant protein expression in transgenic plants by using a double-inducible viral vector. Proc Natl Acad Sci USA 108:14061–14066
Zeitlin L, Pettitt J, Scully C, Bohorova N, Kim D, Pauly M, Hiatt A, Ngo L, Steinkellner H, Whaley KJ, Olinger GG (2011) Enhanced potency of a fucose-free monoclonal antibody being developed as an Ebola virus immunoprotectant. Proc Natl Acad Sci USA 108:20690–20694
Acknowledgements
This work was supported in part by grants from the Ministry of Economy, Trade and Industry (METI) of Japan and New Energy and Industrial Technology Development Organization (NEDO).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Eugenio Benvenuto.
Electronic supplementary material
Below is the link to the electronic supplementary material.
299_2018_2322_MOESM2_ESM.pdf
Fig. S1 GFP expression in CR2Tg or (CR1+2)Tg after vacuum infiltration with the Agrobacterium suspension harboring either CR1+CR3Δ33GFP (a) or only CR3Δ33GFP (b) at 3 dpi. CR2Tg (No. 24) is the transgenic plant expressing CMV RNA 2. (CR1+2)Tg (No. 6) is the transgenic plant expressing both RNA 1 and RNA 2 (PDF 130 KB)
299_2018_2322_MOESM3_ESM.pdf
Fig. S2 Comparison of protein production in plants by the virus-based vector systems. The expression levels of GFP were compared among 4 representative virus vectors (CMV, AMV, CPMV and TMV) (PDF 225 KB)
299_2018_2322_MOESM4_ESM.pdf
Fig. S3 GFP levels in CR1Tg lines after vacuum infiltration with Agrobacterium suspension harboring CR2+CR3Δ33GFP. Tissues were harvested at 3, 5 or 7 dpi. Agroinfiltrated plants were 7 weeks old. Two lines of CR1Tg (Nos. 30 and 79) were used. To estimate levels, a purchased, recombinant GFP was used (PDF 137 KB)
299_2018_2322_MOESM5_ESM.pdf
Fig. S4 IL1-ra levels in a CR1Tg line after vacuum infiltration with Agrobacterium suspension harboring CR2+CR3Δ33IL1-ra. The IL1-ra gene was prepared according to Fukuzawa et al. (2011). Tissues were harvested at 1, 2 or 3 dpi. Agroinfiltrated plants were 7 weeks old. CR1Tg No. 29 line was used. Note that IL1-ra levels were highest at 2 dpi. To estimate levels, a purchased, recombinant IL1-ra protein was used (PDF 58 KB)
Rights and permissions
About this article
Cite this article
Fukuzawa, N., Masuta, C. & Matsumura, T. Rapid transient protein production by the coat protein-deficient cucumber mosaic virus vector: non-packaged CMV system, NoPaCS. Plant Cell Rep 37, 1513–1522 (2018). https://doi.org/10.1007/s00299-018-2322-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-018-2322-5