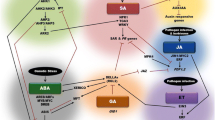
Abstract
The highly coordinated, dynamic nature of growth requires plants to perceive and react to various environmental signals in an interactive manner. Elaborate signaling networks mediate this plasticity in growth and the ability to adapt to changing environmental conditions. The fluctuations of stress-responsive hormones help alter the cellular dynamics and hence play a central role in coordinately regulating the growth responses under stress. Recent experimental data unequivocally demonstrated that interactions among various phytohormones are the rule rather than exception in integrating the diverse input signals and readjusting growth as well as acquiring stress tolerance. The presence of multiple and often redundant signaling intermediates for each phytohormone appears to help in such crosstalk. Furthermore, there are several examples of similar developmental changes occurring in response to distinct abiotic stress signals, which can be explained by the crosstalk in phytohormone signaling. Therefore, in this brief review, we have highlighted the major phytohormone crosstalks with a focus on the response of plants to abiotic stresses. The recent findings have made it increasingly apparent that such crosstalk will also explain the extreme pleiotropic responses elicited by various phytohormones. Indeed, it would not be presumptuous to expect that in the coming years this paradigm will take a central role in explaining developmental regulation.

Similar content being viewed by others
References
Achard P, Cheng H, De Grauwe L, Decat J, Schoutteten H, Moritz T, Van Der Straeten D, Peng J, Harberd NP (2006) Integration of plant responses to environmentally activated phytohormonal signals. Science 311:91–94
Alonso-Ramírez A, Rodríguez D, Reyes D, Angel Jiménez J, Nicolás G, López-Climent M, Gómez-Cadenas A, Nicolás C (2009a) Crosstalk between gibberellins and salicylic acid in early stress responses in Arabidopsis thaliana seeds. Plant Signal Behav 48:750–751
Alonso-Ramírez A, Rodríguez D, Reyes D, Angel Jiménez J, Nicolás G, López-Climent M, Gómez-Cadenas A, Nicolás C (2009b) Evidence for a role of gibberellins in salicylic acid-modulated early plant responses to abiotic stress in Arabidopsis seeds. Plant Physiol 150:1335–1344
Anjum SA, Wang LC, Farooq M, Hussain M, Xue LL, Zou CM (2011) Brassinolide application improves the drought tolerance in maize through modulation of enzymatic antioxidants and leaf gas exchange. J Agron Crop Sci 197:177–185
Bailey-Serres J, Voesenek LACJ (2010) Life in the balance: a signaling network controlling survival of flooding. Curr Opinion Plant Biol 13:489–494
Bajguz A, Hayat S (2009) Effects of brassinosteroids on the plant responses to environmental stresses. Plant Physiol Biochem 47:1–8
Baudouin E (2011) The language of nitric oxide signaling. Plant Biol 13:233–242
Beaudoin N, Serizet C, Gosti F, Giraudat J (2000) Interactions between abscisic acid and ethylene signaling cascades. Plant Cell 12:1103–1115
Benkova E, Hejatko J (2009) Hormone interactions at the root apical meristem. Plant Mol Biol 69:383–396
Breuillin F, Schramm J, Hajirezaei M, Ahkami A, Favre P, Druege U, Hause B, Bucher M, Kretzschmar T, Bossolini E, Kuhlemeier C, Martinoia E, Franken P, Scholz U, Reinhardt D (2010) Phosphate systemically inhibits development of arbuscular mycorrhiza in Petunia hybrida and represses genes involved in mycorrhizal functioning. Plant J 64:1002–1017
Carabelli M, Possenti M, Sessa G, Ciolfi A, Sassi M, Morelli G, Ruberti I (2007) Canopy shade causes a rapid and transient arrest in leaf development through auxin-induced cytokinin oxidase activity. Genes Dev 21:1863–1868
Cho YH, Yoo SD (2009) Emerging complexity of ethylene signal transduction. J Plant Biol 52:283–288
Christmann A, Weiler EW, Steudle E, Grill E (2007) A hydraulic signal in root-to-shoot signaling of water shortage. Plant J 52:167–174
Cui JX, Zhou YH, Ding JG, Xia XJ, Shi K, Chen SC, Asami T, Chen Z, Yu JQ (2011) Role of nitric oxide in hydrogen peroxide-dependent induction of abiotic stress tolerance by brassinosteroids in cucumber. Plant Cell Environ 34:347–358
Cutler SR, Rodriguez PL, Finkelstein RR, Abrams SR (2010) Abscisic acid: emergence of a core signaling network. Annu Rev Plant Biol 61:651–679
Depuydt S, Hardtke CS (2011) Hormone signalling crosstalk in plant growth regulation. Curr Biol 21:R365–R373
Dietz K-J, Vogel MO, Viehhauser A (2010) AP2/EREBP transcription factors are part of gene regulatory networks and integrate metabolic, hormonal and environmental signals in stress acclimation and retrograde signaling. Protoplasma 245:3–14
Domagalska MA, Leyser O (2011) Signal integration in the control of shoot branching. Nature Rev Mol Cell Biol 12:211–221
Dubovskaya LV, Bakakina YS, Kolesneva EV, Sodel DL, McAinsh MR, Hetherington AM, Volotovski ID (2011) cGMP-dependent ABA-induced stomatal closure in the ABA-insensitive Arabidopsis mutant abi1-1. New Phytol 191:57–69
Fleet C, Sun T (2005) A DELLAcate balance: the role of gibberellin in plant morphogenesis. Curr Opin Plant Biol 8:77–85
Fonseca S, Chico JM, Solano R (2009a) The jasmonate pathway: the ligand, the receptor and the core signaling module. Curr Opin Plant Biol 12:539–547
Fonseca S, Chini A, Hamberg M, Adie B, Porzel A, Kramell R, Miersch O, Wasternack C, Solano R (2009b) (+)-7-iso-Jasmonoyl-l-isoleucine is the endogenous bioactive jasmonate. Nature Chem Biol 5:344–350
FTF (2011) Feed the future: global food security research strategy http://www.feedthefuture.gov/sites/default/files/resource/files/FTF_research_strategy.pdf
Fujita Y, Fujita M, Shinozaki K, Yamaguchi-Shinozaki K (2011) ABA-mediated transcriptional regulation in response to osmotic stress in plants. J Plant Res 124:509–525
Gao XH, Xiao SL, Yao QF, Wang YJ, Fu XD (2011) An updated GA signaling ‘Relief of Repression’ regulatory model. Mol Plant 4:601–606
Garcia MJ, Suarez V, Romera FJ, Alcantara E, Perez-Vicente R (2011) A new model involving ethylene, nitric oxide and Fe to explain the regulation of Fe-acquisition genes in Strategy I plants. Plant Physiol Biochem 49:537–544
Gemes K, Poor P, Horvath E, Kolbert Z, Szopko D, Szepesi A, Tari I (2011) Crosstalk between salicylic acid and NaCl-generated reactive oxygen species and nitric oxide in tomato during acclimation to high salinity. Physiol Plant 142:179–192
Golldack D, Li C, Mohan H, Probst N (2013) Gibberellins and abscisic acid signal crosstalk: living and developing under unfavorable conditions. Plant Cell Rep (in this issue). doi:10.1007/s00299-013-1409-2
Gomez-Roldan V, Fermas S, Brewer PB, Puech-Pages V, Dun EA, Pillot JP, Letisse F, Matusova R, Danoun S, Portais JC, Bouwmeester H, Becard G, Beveridge CA, Rameau C, Rochange SF (2008) Strigolactone inhibition of shoot branching. Nature 455:189–194
Gonzalez-Guzman M, Pizzio GA, Antoni R, Vera-Sirera F, Merilob E, Bassel G, Fernández MA, Holdsworth MJ, Perez-Amador MA, Kollist H, Rodriguez PL (2012) Arabidopsis PYR/PYL/RCAR receptors play a major role in quantitative regulation of stomatal aperture and transcriptional response to abscisic acid. Plant Cell 24:2483–2496
Govind G, Seiler C, Wobus U, Sreenivasulu N (2011) Importance of ABA homeostasis under terminal drought stress in regulating grain filling events. Plant Signal Behav 6:1228–1231
Hall AJ, Richards RA (2013) Prognosis for genetic improvement of yield potential and water-limited yield of major grain crops. Field Crops Res 143:18–33
Hao J, Yin Y, Fei S (2013) Brassinosteroid signaling network: Implications on yield and stress tolerance. Plant Cell Rep (in this issue). doi:10.1007/s00299-013-1438-x
Hirano K, Ueguchi-Tanaka M, Matsuoka M (2008) GID1-mediated gibberellin signaling in plants. Trends Plant Sci 13:192–199
Hirayama T, Shinozaki K (2010) Research on plant abiotic stress responses in the post-genome era: past, present, and future. Plant J 61:1041–1052
Hossain MA, Munemasa S, Uraji M, Nakamura Y, Mori IC, Murata Y (2011) Involvement of endogenous abscisic acid in methyl jasmonate-induced stomatal closure in Arabidopsis. Plant Physiol 156:430–438
Hou X, Ding L, Yu H (2013) Crosstalk between GA and JA signaling mediates plant growth and defense. Plant Cell Rep (in this issue). doi:10.1007/s00299-013-1423-4
Huang J, Yang M, Liu P, Yang G, Wu C, Zheng CC (2009) GhDREB1 enhances abiotic stress tolerance, delays GA-mediated development and represses cytokinin signaling in transgenic Arabidopsis. Plant, Cell Environ 32:1132–1145
IPCC (2007) Summary for Policymakers. In: Parry ML, Canziani OF, Palutikof JP, van der Linden PJ, Hanson CE (Secondary Authors), Climate Change 2007: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change, Cambridge, UK
Iqbal N, Nazar R, Khan MIR, Masood A, Khan NA (2011) Role of gibberellins in regulation of source–sink relations under optimal and limiting environmental conditions. Curr Sci 100:998–1007
Ivanchenko MG, Muday GK, Dubrovsky JG (2008) Ethylene-auxin interactions regulate lateral root initiation and emergence in Arabidopsis thaliana. Plant J 55:335–347
Ji X, Dong B, Shiran B, Talbot MJ, Edlington JE, Hughes T, White RG, Gubler F, Dolferus R (2011) Control of abscisic acid catabolism and abscisic acid homeostasis is important for reproductive stage stress tolerance in cereals. Plant Physiol 156:647–662
Joshi-Saha A, Valon C, Leung J (2011) Abscisic acid signal off the STARTing block. Mol Plant 4:562–580
Kanyuka K, Praekelt U, Franklin KA, Billingham OE, Hooley R, Whitelam GC, Halliday KJ (2010) Mutations in the huge Arabidopsis gene BIG affect a range of hormone and light responses. Plant J 35:57–70
Kapulnik Y, Resnick N, Mayzlish-Gati E, Kaplan Y, Wininger S, Hershenhorn J, Koltai H (2011) Strigolactones interact with ethylene and auxin in regulating root-hair elongation in Arabidopsis. J Exp Bot 62:2915–2924
Kim EH, Kim YS, Park SH, Koo YJ, Choi YD, Chung YY, Lee IJ, Kim JK (2009) Methyl jasmonate reduces grain yield by mediating stress signals to alter spikelet development in rice. Plant Physiol 149:1751–1760
Kim YY, Jung KW, Yoo KS, Jeung JU, Shin JS (2011) A stress-responsive caleosin-like protein, AtCLO4, acts as a negative regulator of ABA responses in Arabidopsis. Plant Cell Physiol 52:874–884
Kline KG, Barrett-Wilt GA, Sussman MR (2010) In planta changes in protein phosphorylation induced by the plant hormone abscisic acid. Proc Natl Acad Sci USA 107:15986–15991
Klingler JP, Batelli G, Zhu JK (2010) ABA receptors: the START of a new paradigm in phytohormone signalling. J Exp Bot 61:3199–3210
Koltai H (2011) Strigolactones are regulators of root development. New Phytol 190:545–549
Krouk G, Ruffel S, Gutierrez RA, Gojon A, Crawford NM, Coruzzi GM, Lacombe B (2011) A framework integrating plant growth with hormones and nutrients. Trends Plant Sci 16:178–182
Lackman P, González-Guzmán M, Tilleman S, Carqueijeiro I, Pérez AC, Moses T, Seo M, Kanno Y, Häkkinen ST, Van Montagu MCE, Thevelein JM, Maaheimo H, Oksman-Caldentey KM, Rodriguez PL, Rischer H, Goossens A (2011) Jasmonate signaling involves the abscisic acid receptor PYL4 to regulate metabolic reprogramming in Arabidopsis and tobacco. Proc Natl Acad Sci USA 108:5891–5896
Li J, Jin H (2007) Regulation of brassinosteroid signaling. Trends Plant Sci 12:37–41
Li H, Jiang H, Bu Q, Zhao Q, Sun J, Xie Q, Li C (2011) The Arabidopsis RING finger E3 ligase RHA2b acts additively with RHA2a in regulating abscisic acid signaling and drought response. Plant Physiol 156:550–563
Lin Z, Ho C-W, Grierson D (2009) AtTRP1 encodes a novel TPR protein that interacts with the ethylene receptor ERS1 and modulates development in Arabidopsis. J Exp Bot 60:3697–3714
Linkies A, Leubner-Metzger G (2012) Beyond gibberellins and abscisic acid: how ethylene and jasmonates control seed germination. Plant Cell Rep 31:253–270
Lopez-Raez JA, Kohlen W, Charnikhova T, Mulder P, Undas AK, Sergeant MJ, Verstappen F, Bugg TDH, Thompson AJ, Ruyter-Spira C, Bouwmeester H (2010) Does abscisic acid affect strigolactone biosynthesis? New Phytol 187:343–354
Lyons R, Manners JM, Kazan K (2013) Jasmonate biosynthesis and signaling in monocots: a comparative overview. Plant Cell Rep 32:815–827
Ma Y, Szostkiewicz I, Korte A, Moes D, Yang Y, Christman A, Grill E (2009) Regulators of PP2C phosphatase activity function as abscisic acid sensors. Science 324:1064–1068
Martin-Rejano EM, Camacho-Cristobal JJ, Herrera-Rodriguez BM, Rexach J, Navarro-Gochicoa TM, Gonzalez-Fontes A (2011) Auxin and ethylene are involved in the responses of root system architecture to low boron supply in Arabidopsis seedlings. Physiol Plant 142:170–178
Morant M, Ekstrom C, Ulvskov P, Kristensen C, Rudemo M, Olsen CE, Hansen J, Jorgensen K, Jorgensen B, Moller BL, Bak S (2010) Metabolomic, transcriptional, hormonal, and signaling crosstalk in Superroot2. Mol Plant 3:192–211
Nakashima K, Yamaguchi-Shinozaki K (2013) ABA signaling in stress-response and seed development. Plant Cell Rep (in this issue). doi:10.1007/s00299-013-1418-1
Negi S, Sukumar P, Liu X, Cohen JD, Muday GK (2010) Genetic dissection of the role of ethylene in regulating auxin-dependent lateral and adventitious root formation in tomato. Plant J 61:3–15
Nemhauser JL, Hong F, Chory J (2006) Different plant hormones regulate similar processes through largely nonoverlapping transcriptional responses. Cell 126:467–475
Nishiyama R, Watanabe Y, Fujita Y, Dung Tien L, Kojima M, Werner T, Vankova R, Yamaguchi-Shinozaki K, Shinozaki K, Kakimoto T, Sakakibara H, Schmuelling T, Lam-Son Phan T (2011) Analysis of cytokinin mutants and regulation of cytokinin metabolic genes reveals important regulatory roles of cytokinins in drought, salt and abscisic acid responses, and abscisic acid biosynthesis. Plant Cell 23:2169–2183
Niu Y, Jin C, Jin G, Zhou Q, Lin X, Tang C, Zhang Y (2011) Auxin modulates the enhanced development of root hairs in Arabidopsis thaliana (L.) Heynh. under elevated CO2. Plant Cell Environ 34:1304–1317
Nomura S, Nakashima H, Mizutani M, Takikawa H, Sugimoto Y (2013) Structural requirements of strigolactones for germination induction and inhibition of Striga gesnerioides seeds. Plant Cell Rep 32:829–838
Pareek A, Sopory SK, Bohnert HJ, Govindjee (eds) (2010) Abiotic stress adaptation in plants. Springer, Dordrecht
Park SY, Fung P, Nishimura N, Jensen DR, Fujii H, Zhao Y, Lumba S, Santiago J, Rodrigues A, Chow TF, Alfred SE, Bonetta D, Finkelstein R, Provart NJ, Desveaux D, Rodriguez PL, McCourt P, Zhu JK, Schroeder JI, Volkman BF, Cutler SR (2009) Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science 324:1068–1071
Peleg Z, Blumwald E (2011) Hormone balance and abiotic stress tolerance in crop plants. Curr Opin Plant Biol 14:290–295
Peleg Z, Reguera M, Tumimbang E, Walia H, Blumwald E (2011) Cytokinin-mediated source/sink modifications improve drought tolerance and increase grain yield in rice under water-stress. Plant Biotech J 9:747–758
Pinheiro C, Chaves MM (2011) Photosynthesis and drought: can we make metabolic connections from available data? J Exp Bot 62:869–882
Pospisilova J (2003) Participation of phytohormones in the stomatal regulation of gas exchange during water stress. Biol Plant 46:491–506
Pospisilova J, Vagner M, Malbeck J, Travniakova A, Batkova P (2005) Interactions between abscisic acid and cytokinins during water stress and subsequent rehydration. Biologia Plant 49:533–540
Potters G, Pasternak TP, Guisez Y, Jansen MAK (2009) Different stresses, similar morphogenic responses: integrating a plethora of pathways. Plant Cell Environ 32:158–169
Qin F, Shinozaki K, Yamaguchi-Shinozaki K (2011) Achievements and challenges in understanding plant abiotic stress responses and tolerance. Plant Cell Physiol 52:1569–1582
Raghavendra AS, Gonugunta VK, Christmann A, Grill E (2010) ABA perception and signalling. Trends Plant Sci 15:395–401
Rani K, Zwanenburg B, Sugimoto Y, Yoneyama K, Bouwmeester HJ (2008) Biosynthetic considerations could assist the structure elucidation of host plant produced rhizosphere signalling compounds (strigolactones) for arbuscular mycorrhizal fungi and parasitic plants. Plant Physiol Biochem 46:617–626
Robert HS, Friml J (2009) Auxin and other signals on the move in plants. Nat Chem Biol 5:325–332
RoyChoudhury A, Paul S, Basu S (2013) Crosstalk between abscisic acid-dependent and abscisic acid-independent pathways during abiotic stress. Plant Cell Rep (in this issue). doi:10.1007/s00299-013-1414-5
Sakuma Y, Liu Q, Dubouzet JG, Abe H, Shinozaki K, Yamaguchi-Shinozaki K (2002) DNA-binding specificity of the ERF/AP2 domain of Arabidopsis DREBs, transcription factors involved in dehydration- and cold-inducible gene expression. Biochem Biophys Res Commun 290:998–1009
Santino A, Taurino M, De Domenico S, Bonsegna S, Poltronieri P, Pastor V, Flors V (2013) Jasmonate signaling in plant development and defense response to multiple (a)biotic stresses. Plant Cell Rep (in this issue). doi:10.1007/s00299-013-1441-2
Santner A, Calderon-Villalobos LIA, Estelle M (2009) Plant hormones are versatile chemical regulators of plant growth. Nature Chem Biol 5:301–307
Schroeder F, Lisso J, Muessig C (2011) EXORDIUM-LIKE1 promotes growth during low carbon availability in Arabidopsis. Plant Physiol 156:1620–1630
Seiler C, Harshavardhan VT, Rajesh K, Reddy PS, Strickert M, Rolletschek H, Scholz U, Wobus U, Sreenivasulu N (2011) ABA biosynthesis and degradation contributing to ABA homeostasis during barley seed development under control and terminal drought-stress conditions. J Exp Bot 62:2615–2632
Seki M, Ishida J, Narusaka M, Fujita M, Nanjo T, Umezawa T, Kamiya A, Nakajima M, Enju A, Sakurai T, Satou M, Akiyama K, Yamaguchi-Shinozaki K, Carninci P, Kawai J, Hayashizaki Y, Shinozaki K (2002) Monitoring the expression pattern of around 7,000 Arabidopsis genes under ABA treatments using a full-length cDNA microarray. Funct Integr Genomics 2:282–291
Seo M, Koshiba T (2011) Transport of ABA from the site of biosynthesis to the site of action. J Plant Res 124:501–507
Shahid MA, Pervez MA, Balal RM, Mattson NS, Rashid A, Ahmad R, Ayyub CM, Abbas T (2011) Brassinosteroid (24-epibrassinolide) enhances growth and alleviates the deleterious effects induced by salt stress in pea (Pisum sativum L.). Aust J Crop Sci 5:500–510
Siddiqui MH, Al-Whaibi MH, Basalah MO (2010) Role of nitric oxide in tolerance of plants to abiotic stress. Protoplasma 248:447–455
Simontacchi M, García-Mata C, Bartoli CG, Santa-María GE Lamattina L (2013) Nitric oxide as a key component in hormone-regulated processes. Plant Cell Rep 32:853–866
Singh R, Jwa NS (2013) The rice MAPKK-MAPK interactome: the biological significance of MAPK components in hormone signal transduction. Plant Cell Rep 32:923–931
Skirycz A, Claeys H, De Bodt S, Oikawa A, Shinoda S, Andriankaja M, Maleux K, Eloy NB, Coppens F, Yoo S-D, Saito K, Inze D (2011a) Pause-and-stop: the effects of osmotic stress on cell proliferation during early leaf development in Arabidopsis and a role for ethylene signaling in cell cycle arrest. Plant Cell 23:1876–1888
Skirycz A, Vandenbroucke K, Clauw P, Maleux K, De Meyer B, Dhondt S, Pucci A, Gonzalez N, Hoeberichts F, Tognetti VB, Galbiati M, Tonelli C, Van Breusegem F, Vuylsteke M, Inze D (2011b) Survival and growth of Arabidopsis plants given limited water are not equal. Nat Biotechol 29:212–214
Sreenivasulu N, Sopory SK, Kavi Kishor PB (2007) Deciphering the regulatory mechanisms of abiotic stress tolerance in plants by genomic approaches. Gene 388:1–13
Sreenivasulu N, Harshavardhan VT, Govind G, Seiler C, Kohli A (2012) Contrapuntal role of ABA: does it mediate stress tolerance or plant growth retardation under long-term drought stress? Gene 506:265–273
Stamm P, Kumar PP (2010) The phytohormone signal network regulating elongation growth during shade avoidance. J Exp Bot 61:2889–2903
Stamm P, Kumar PP (2013) Auxin and gibberellin responsive Arabidopsis SMALL AUXIN UP RNA36 regulates hypocotyl elongation in the light. Plant Cell Rep 32:759–769
Stamm P, Ravindran P, Mohanty B, Tan EL, Yu H, Kumar PP (2012a) Insights into the molecular mechanism of RGL2-mediated inhibition of seed germination in Arabidopsis thaliana. BMC Plant Biol 12:179. doi:10.1186/1471-2229-12-179 (http://www.biomedcentral.com/1471-2229/12/179)
Stamm P, Verma V, Ramamoorthy R, Kumar PP (2012b) Manipulation of plant architecture to enhance lignocellulosic biomass. AoB Plants. doi:10.1093/aobpla/pls026
Stone SL, Williams LA, Farmer LM, Vierstra RD, Callis J (2006) KEEP ON GOING, a RING E3 ligase essential for Arabidopsis growth and development, is involved in abscisic acid signaling. Plant Cell 18:3415–3428
Strader LC, Chen GL, Bartel B (2010) Ethylene directs auxin to control root cell expansion. Plant J 64:874–884
Sugimoto Y, Ali AM, Yabuta S, Kinoshita H, Inanaga S, Itai A (2003) Germination strategy of Striga hermonthica involves regulation of ethylene biosynthesis. Physiol Plant 119:137–145
Tran LS, Urao T, Qin F, Maruyama K, Kakimoto T, Shinozaki K, Yamaguchi-Shinozaki K (2007) Functional analysis of AHK1/ATHK1 and cytokinin receptor histidine kinases in response to abscisic acid, drought, and salt stress in Arabidopsis. Proc Natl Acad Sci USA 104:20623–20628
Ueguchi-Tanaka M, Ashikari M, Nakajima M, Itoh H, Katoh E, Kobayashi M, Chow TY, Hsing YIC, Kitano H, Yamaguchi I, Matsuoka M (2005) GIBBERELLIN INSENSITIVE DWARF1 encodes a soluble receptor for gibberellin. Nature 437:693–698
Ueguchi-Tanaka M, Nakajima M, Motoyuki A, Matsuoka M (2007) Gibberellin receptor and its role in gibberellin signaling in plants. Annu Rev Plant Biol 58:183–198
Umehara M, Hanada A, Magome H, Takeda-Kamiya N, Yamaguchi S (2010) Contribution of strigolactones to the inhibition of tiller bud outgrowth under phosphate deficiency in rice. Plant Cell Physiol 51:1118–1126
Umezawa T, Sugiyama N, Mizoguchi M, Hayashi S, Myouga F, Yamaguchi-Shinozaki K, Ishihama Y, Hirayama T, Shinozaki K (2009) Type 2C protein phosphatases directly regulate abscisic acid-activated protein kinases in Arabidopsis. Proc Natl Acad Sci USA 106:17588–17593
Umezawa T, Hirayama T, Kuromori T, Shinozaki K (2011) The regulatory networks of plant responses to abscisic acid. In: Turkan I (ed), Plant responses to drought and salinity stress: developments in a post-genomic era. Adv Bot Res 57:201–248
Vercruyssen L, Gonzalez N, Werner T, Schmuelling T, Inze D (2011) Combining enhanced root and shoot growth reveals cross talk between pathways that control plant organ size in Arabidopsis. Plant Physiol 155:1339–1352
Walia H, Wilson C, Wahid A, Condamine P, Cui X, Close TJ (2006) Expression analysis of barley (Hordeum vulgare L.) during salinity stress. Funct Integr Genomics 6:143–156
Wang F, Cui X, Sun Y, Dong CH (2013) Ethylene signaling and regulation in plant growth and stress responses. Plant Cell Rep (in this issue). doi:10.1007/s00299-013-1421-6
Wasternack C, Kombrink E (2010) Jasmonates: structural requirements for lipid-derived signals active in plant stress responses and development. ACS Chem Biol 5:63–77
Weiss D, Ori N (2007) Mechanisms of crosstalk between gibberellin and other hormones. Plant Physiol 144:1240–1246
Wilkinson S, Davies WJ (2010) Drought, ozone, ABA and ethylene: new insights from cell to plant to community. Plant Cell Environ 33:510–525
Wolters H, Jürgens G (2009) Survival of the flexible: hormonal growth control and adaptation in plant development. Nat Rev Genet 10:305–317
Xi W, Liu C, Hou X, Yu H (2010) MOTHER OF FT AND TFL1 regulates seed germination through a negative feedback loop modulating ABA signaling in Arabidopsis. Plant Cell 22:1733–1748
Xu K, Xu X, Fukao T, Canlas P, Maghirang-Rodriguez R, Heuer S, Ismail AM, Bailey-Serres J, Ronald PC, Mackill DJ (2006) SUB1A is an ethylene-response-factor-like gene that confers submergence tolerance to rice. Nature 442:705–708
Xu J, Wang W, Yin H, Liu X, Sun H, Mi Q (2010) Exogenous nitric oxide improves antioxidative capacity and reduces auxin degradation in roots of Medicago truncatula seedlings under cadmium stress. Plant Soil 326:321–330
Xu J, Wang W, Sun J, Zhang Y, Ge Q, Du L, Yin H, Liu X (2011a) Involvement of auxin and nitric oxide in plant Cd-stress responses. Plant Soil 346:107–119
Xu ZS, Chen M, Li LC, Ma YZ (2011b) Functions and application of the AP2/ERF transcription factor family in crop improvement. J Integr Plant Biol 53:570–585
Xu ZY, Kim DH, Hwang I (2013) ABA homeostasis and signaling involving multiple subcellular compartments and multiple receptors. Plant Cell Rep 32:807–813
Yan J, Zhang C, Gu M, Bai Z, Zhang W, Qi T, Cheng Z, Peng W, Luo H, Nan F, Wang Z, Xie D (2009) The Arabidopsis CORONATINE INSENSITIVE1 protein is a jasmonate receptor. Plant Cell 21:2220–2236
Yang X, Yang YN, Xue LJ, Zou MJ, Liu JY, Chen F, Xue HW (2011a) Rice ABI5-Like1 regulates abscisic acid and auxin responses by affecting the expression of ABRE-containing genes. Plant Physiol 156:1397–1409
Yang CJ, Zhang C, Lu YN, Jin JQ, Wang XL (2011b) The mechanisms of brassinosteroids’ action: from signal transduction to plant development. Mol Plant 4:588–600
Yuan K, Rashotte AM, Wysocka-Diller JW (2011) ABA and GA signaling pathways interact and regulate seed germination and seedling development under salt stress. Acta Physiol Plant 33:261–271
Zhang X, Garreton V, Chua NH (2005) The AIP2 E3 ligase acts as a novel negative regulator of ABA signaling by promoting ABI3 degradation. Genes Dev 19:1532–1543
Zhang S, Cai Z, Wang X (2009a) The primary signaling outputs of brassinosteroids are regulated by abscisic acid signaling. Proc Natl Acad Sci USA 106:4543–4548
Zhang Z, Zhang H, Quan R, Wang XC, Huang R (2009b) Transcriptional regulation of the ethylene response factor LeERF2 in the expression of ethylene biosynthesis genes controls ethylene production in tomato and tobacco. Plant Physiol 150:365–377
Zhang A, Zhang J, Zhang J, Ye N, Zhang H, Tan M, Jiang M (2011) Nitric oxide mediates brassinosteroid-induced ABA biosynthesis involved in oxidative stress tolerance in maize leaves. Plant Cell Physiol 52:181–192
Zheng C, Jiang D, Dai T, Jing Q, Cao W (2010) Effects nitroprusside, a nitric oxide donor, on carbon and nitrogen metabolism and the activity of the antioxidation system in wheat seedlings under salt stress. Acta Ecologica Sinica 30:1174–1183
Acknowledgments
We thank Dr. R. Ramamoorthy for technical assistance in preparing the illustration material and Dr. Vivek Verma for critical reading of the manuscript. Research work in PPK’s laboratory is supported by the National Research Foundation, Singapore under its Competitive Research Program (CRP Award No. NRF-CRP 7-2010-02).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by N. Stewart.
A contribution to the Special Issue: Plant Hormone Signaling.
Rights and permissions
About this article
Cite this article
Kohli, A., Sreenivasulu, N., Lakshmanan, P. et al. The phytohormone crosstalk paradigm takes center stage in understanding how plants respond to abiotic stresses. Plant Cell Rep 32, 945–957 (2013). https://doi.org/10.1007/s00299-013-1461-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-013-1461-y