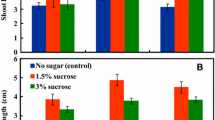
Abstract
Soybean (Glycine max L. Merrill) somatic embryos have been useful for assaying seed-specific traits prior to plant recovery. Such traits could be assessed more accurately if somatic embryos more closely mimicked seed development. Amino acid supplements, carbon source, and abscisic acid and basal salt formulations were tested in an effort to modify existing soybean embryogenesis histodifferentiation/maturation media to further normalize the development of soybean somatic embryos. The resultant liquid medium, referred to as soybean histodifferentiation and maturation medium (SHaM), consists of FNL basal salts, 3% sucrose, 3% sorbitol, filter-sterilized 30 mM glutamine and 1 mM methionine. SHaM-derived somatic embryos are more similar to seed in terms of protein and fatty acid/lipid composition, and conversion ability, than somatic embryos obtained from traditional soybean histodifferentiation and maturation media.



Similar content being viewed by others
References
Anandarajah K, McKersie BD (1990) Enhanced vigor of dry somatic embryos of Medicago sativa L. with increased sucrose. Plant Sci 71:261–266
Bailey MA, Boerma HR, Parrott WA (1993) Genotype effects on proliferative embryogenesis and plant regeneration of soybean. In Vitro Cell Dev Biol 29P:102–108
Blackman SA, Obendorf RL, Leopold AC (1992) Maturation proteins and sugars in desiccation tolerance of developing soybean seeds. Plant Physiol 100:225–230
Blackman SA, Obendorf RL, Leopold AC (1995) Desiccation tolerance in developing soybean seeds: the role of stress proteins. Physiol Plant 93:630–638
Blackman SA, Wettlaufer SH, Obendorf RL, Leopold AC (1991) Maturation proteins associated with desiccation tolerance in soybean. Plant Physiol 96:868–874
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brisibe EA, Miyake H (1994) Abscisic acid and high osmoticum regulation of development and storage reserve accumulation in sugarcane somatic embryos. Jpn J Crop Sci 63:689–698
Cahoon EB, Carlson TJ, Ripp KG, Schweiger BJ, Cook GA, Hall SE, Kinney AJ (1999) Biosynthetic origin of conjugated double bonds: production of fatty acid components of high-value drying oils in transgenic soybean embryos. Proc Natl Acad Sci USA 96:12935–12940
Cahoon EB, Marillia E-F, Stecca KL, Hall SE, Taylor DC, Kinney AJ (2000) Production of fatty acid components of meadowfoam oil in somatic soybean embryos. Plant Physiol 124:243–251
Cho M-J, Widholm JM, Vodkin LO (1995) Cassettes for seed-specific expression tested in transformed embryogenic cultures of soybean. Plant Mol Biol Rep 13:255–269
Coker GTI, Garbow JR, Schaefer J (1987) 15N and13C NMR determination of methionine metabolism in developing soybean cotyledons. Plant Physiol 83:698–702
Dyer DJ, Cotterman CD, Cotterman JC (1987) Comparison of in situ and in vitro regulation of soybean seed growth and development. Plant Physiol 84:298–303
Egli DB (1990) Seed water relations and the regulation of the duration of seed growth in soybean. J Exp Bot 41:243–248
Finer JJ, Nagasawa A (1988) Development of an embryogenic suspension culture of soybean [Glycine max (L.) Merrill]. Plant Cell Tissue Organ Cult 15:125–136
Finkelstein R, Somerville C (1989) Abscisic acid or high osmoticum promote accumulation of long-chain fatty acids in developing embryos of Brassica napus. Plant Sci 61:213–217
Fujii JAA, Slade D, Olsen R, Ruzin SE, Redenbaugh K (1990) Alfalfa somatic embryo maturation and conversion to plants. Plant Sci 72:93–100
Herman EM, Helm RM, Jung R, Kinney AJ (2003) Genetic modification removes an immunodominant allergen from soybean. Plant Physiol 132:36–43
Holbrook LA, Magus JR, Taylor DC (1992) Abscisic acid inductin of elongase activity, biosynthesis and accumulation of very long chain monounsaturated fatty acids and oil body proteins in microspore-derived embryos of Brassica napus L. cv. Reston. Plant Sci 84:99–115
Horbowicz M, Obendorf RL, McKersie BD, Viands DR (1995) Soluble saccharides and cyclitols in alfalfa (Medicago sativa L.) somatic embryos, leaflets, and mature seeds. Plant Sci 109:191–198
Huang B, Bird S, Kemble R, Miki B, Keller W (1991) Plant regeneration from microspore-derived embryos of Brassica napus: effect of embryo age, culture temperature, osmotic pressure, and abscisic acid. In Vitro Cell Dev Biol Plant 27P:28–31
Kermode AR (1995) Regulatory mechanisms in the transition from seed development to germination: interactions between the embryo and the seed environment. In: Kigel J, Galili G (eds) Seed development and germination. Dekker, New York, pp 273–332
Lai F-M, Lecouteux CG, McKersie BD (1995) Germination of alfalfa (Medicago sativa L.) seeds and desiccated somatic embryos I. Mobilization of storage reserves. J Plant Physiol 145:507–513
Lai F-M, McKersie BD (1993) Effect of nutrition on maturation of alfalfa (Medicago sativa L.) somatic embryos. Plant Sci 91:87–95
Lai F-M, McKersie BD (1994b) Regulation of starch and protein accumulation in alfalfa (Medicago sativa L.) somatic embryos. Plant Sci 100:211–219
Lai F-M, McKersie BD (1994a) Regulation of storage protein synthesis by nitrogen and sulfur nutrients in alfalfa (Medicago sativa L.) somatic embryos. Plant Sci 103:209–221
Lai F, Senaratna T, McKersie BD (1992) Glutamine enhances storage protein synthesis in Medicago sativa L. somatic embryos. Plant Sci 87:69–77
Lippmann B, Lippmann G (1993) Soybean embryo culture: factors influencing plant recovery from isolated embryos. Plant Cell Tissue Organ Cult 32:83–90
Liu W, Torisky RS, McAllister KP, Avdiushko S, Hildebrand DF, Collins GB (1996) Somatic embryo cycling: evaluation of a novel transformation and assay system for seed-specific gene expression in soybean. Plant Cell Tissue Organ Cult 47:33–42
Marillia EF, Giblin EM, Covello PS, Taylor DC (2002) Expression of meadowfoam Des5 and FAE1 genes in yeast and in transgenic soybean somatic embryos, and their roles in fatty acid modification. Plant Physiol Biochem 40:821–828
Mazur B, Krebbers E, Tingey S (1999) Gene discovery and product development for grain quality traits. Science 285:372–375
Merkle SA, Parrott WA, Flinn BS (1994) Morphogenic aspects of somatic embryogenesis. In: Thorpe TA (ed) Somatic embryogenesis. CRC, Boca Raton, Fla., pp 155–203
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning—a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
Samoylov VM, Tucker DM, Parrott WA (1998a) A liquid medium-based protocol for rapid regeneration from embryogenic soybean cultures. Plant Cell Rep 18:49–54
Samoylov VM, Tucker DM, Parrott WA (1998b) Soybean [Glycine max (L.) Merrill] embryogenic cultures: the role of sucrose and total nitrogen content on proliferation. In Vitro Cell Dev Biol Plant 34:8–13
Saravitz CH, Raper CD Jr (1995) Responses to sucrose and glutamine by soybean embryos grown in vitro. Physiol Plant 93:799–805
Stacey G, Vodkin L, Parrott WA, Shoemaker RC (2004) National science foundation-sponsored workshop report. Draft plan for soybean genomics. Plant Physiol 135:59–70
TeKrony DM, Egli DB, Balles J, Pfeiffer T, Fellows RJ (1979) Physiological maturity in soybean. Agron J 71:771–775
Thompson JF, Madison JT, Muenster A-ME (1977) In vitro culture of immature cotyledons of soya bean (Glycine max L. Merr.). Ann Bot 41:29–39
Tian LN, Brown DCW (2000) Improvement of soybean somatic embryo development and maturation by abscisic acid treatment. Can J Plant Sci 80:721–276
Wilen RW, Mandel RM, Pharis RP, Holbrook LA, Moloney MM (1990) Effects of abscisic acid and high osmoticum on storage protein gene expression in microspore embryos of Brassica napus. Plant Physiol 94:875–881
Walker DR, Parrott WA (2001) Effect of polyethylene glycol and sugar alcohols on soybean somatic embryo germination and conversion. Plant Cell Tissue Organ Cult 64:55–62
Xu N, Coulter KM, Bewley JD (1990) Abscisic acid and osmoticum prevent germination of developing alfalfa embryos, but only osmoticum maintains the synthesis of developmental proteins. Planta 182:382–390
Zimmerman JL (1993) Somatic embryogenesis: a model for early development in higher plants. Plant Cell 5:1411–1423
Acknowledgements
This work was funded by a gift from Pioneer HiBred, International, a grant from the United Soybean Board, and from state and federal monies allocated to the Georgia Agricultural Experiment Stations
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by P. Ozias-Akins
Rights and permissions
About this article
Cite this article
Schmidt, M.A., Tucker, D.M., Cahoon, E.B. et al. Towards normalization of soybean somatic embryo maturation. Plant Cell Rep 24, 383–391 (2005). https://doi.org/10.1007/s00299-005-0950-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-005-0950-z