Abstract
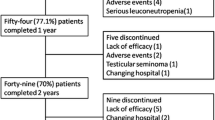
Despite the progress in the treatment of ankylosing spondylitis (AS), a significant number of patients do not achieve low disease activity (LDA). The aim of the study is to estimate the size of unmet needs in the treatment of AS in a long-term observational study. Between January 2003 and December 2017, 220 patients with radiographic SpA were evaluated fulfilling the ASAS criteria. They were followed up at predefined times and were naive to biological treatment with anti-tumor necrosis factor agents (anti-TNFs) and the interleukin (IL)-17 inhibitor. NSAIDs, all anti-TNFs and the IL-17 inhibitor secukinumab were used according to the European, United States and Canadian guidelines for AS. During follow-up, several clinical parameters including disease activity scores were recorded. All 220 patients had an active disease and received at least two NSAIDs for 3 months. The anti-TNF of first choice was infliximab—51%, followed by adalimumab—27% and etanercept—22%. During follow-up, 22 patients were excluded from the study (18 lost, 4 never received anti-TNF due to comorbidities). From the rest (198), 12 did not receive anti-TNFs (8 due to sustained LDA on NSAIDs solely and 4 due to treatment denial). Finally, 186 (94%) were treated with anti-TNFs demonstrating sustained long-term LDA. However, 16 patients never achieved LDA despite they received two or three anti-TNFs or the IL-17 inhibitor. Thus, a total of 20 (10.1%) patients never achieved LDA. This is the first study aiming to estimate the gap and the size of unmet needs in AS patients using the international guidelines and recommendations for AS treatment, which is 10.1%.


Similar content being viewed by others
References
Taurog JD, Chhabra A, Colbert RA (2016) ankylosing spondylitis and axial spondyloarthritis. N Engl J Med 374:2563–2574
Alamanos Y, Papadopoulos NG, Voulgari PV, Karakatsanis A, Siozos C, Drosos AA (2004) Epidemiology of ankylosing spondylitis in Northwest Greece, 1983–2002. Rheumatology 43:615–618
Reveille JD, Witter JP, Weisman MH (2012) Prevalence of axial spondyloarthritis in the US: estimates from a cross-sectional survey. Arthritis Care Res (Hoboken) 64:905–910
Stolwijk C, van Onna M, Boonen A, van Tubergen A (2016) Global prevalence of spondyloarthritis: a systematic review and meta-regression analysis. Arthritis Care Res (Hoboken) 68:1320–1331
van Hoeven L, Luime J, Han H, Vergouwe Y, Weel A (2014) Identifying axial spondyloarthritis in Dutch primary care patients, ages 20–45 years, with chronic low back pain. Arthritis Care Res (Hoboken) 66:446–453
Rudwaleit M, van der Heijde D, Landewé R et al (2009) The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis 68:777–783
Rudwaleit M, van der Heijde D, Landewé R et al (2011) The Assessment of SpondyloArthritis International Society classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general. Ann Rheum Dis 70:25–31
Maksymowych WP, Inman RD, Gladman D et al (2003) Spondyloarthritis Research Consortium of Canada (SPARCC). Canadian Rheumatology Association Consensus on the use of anti-tumor necrosis factor-alpha directed therapies in the treatment of spondyloarthritis. J Rheumatol 30:1356–1363
Glintborg B, Sørensen IJ, Østergaard M et al (2017) Ankylosing spondylitis versus nonradiographic axial spondyloarthritis: comparison of tumor necrosis factor inhibitor effectiveness and effect of HLA-B27 status. an observational cohort study from the Nationwide DANBIO Registry. J Rheumatol 44:59–69
Flouri ID, Markatseli TE, Boki KA et al (2018) Comparative analysis and predictors of 10-year tumor necrosis factor inhibitors drug survival in patients with spondyloarthritis: first-year response predicts longterm drug persistence. J Rheumatol 45:785–794
Wang R, Dasgupta A, Ward MM (2018) Comparative efficacy of tumor necrosis factor-α inhibitors in ankylosing spondylitis: a systematic review and bayesian network metaanalysis. J Rheumatol 45:481–490
Duarte JH (2016) Spondyloarthropathies: IL-17A blockade ameliorates ankylosing spondylitis. Nat Rev Rheumatol 12:72
Braun J, Baraliakos X, Deodhar A, MEASURE 1 study group et al (2017) Effect of secukinumab on clinical and radiographic outcomes in ankylosing spondylitis: 2-year results from the randomised phase III MEASURE 1 study. Ann Rheum Dis 76:1070–1077
Baraliakos X, Kivitz AJ, Deodhar AA, MEASURE 1 study group et al (2018) Long-term effects of interleukin-17A inhibition with secukinumab in active ankylosing spondylitis: 3-year efficacy and safety results from an extension of the Phase 3 MEASURE 1 trial. Clin Exp Rheumatol 36:50–55
Tramér MR, Moore RA, Reynolds DJ et al (2000) Quantitative estimation of rare adverse events which follow a biological progression: anew model applied to chronic NSAID use. Pain 85(1–2):169–182
Singh G, Triadafilopoulos G (1999) Epidemiology of NSAID induced gastrointestinal complications. J Rheumatol Suppl 56:18–24
Braun J, van den Berg R, Baraliakos X et al (2011) 2010 update of the ASAS/EULAR recommendations for the management of ankylosing spondylitis. Ann Rheum Dis 70:896–904
van der Heijde D, Ramiro S, Landewé R et al (2017) 2016 Update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann Rheum Dis 76:978–991
Ward MM, Deodhar A, Akl EA et al (2016) American College of Rheumatology/ Spondylitis Association of America/Spondyloarthritis Research and Treatment Network 2015 recommendations for the treatment of ankylosing spondylitis and nonradiographic axial spondyloarthritis. Arthritis Rheumatol 68:282–298
Rohekar S, Chan J, Tse SM et al (2015) 2014 Update of the Canadian Rheumatology Association/Spondyloarthritis Research Consortium of Canada treatment recommendations for the management of spondyloarthritis. II. specific management recommendations. J Rheumatol 42:665–681
Calin A, Garrett S, Whitelock H et al (1994) A new approach to defining functional ability in ankylosing spondylitis: the development of the Bath Ankylosing Spondylitis Functional Index. J Rheumatol 21:2281–2285
Lukas C, Landewé R, Sieper J, Assessment of SpondyloArthritis international Society et al (2009) Development of an ASAS-endorsed disease activity score (ASDAS) in patients with ankylosing spondylitis. Ann Rheum Dis 68:18–24
Machado P, Landewé R, Lie E, Assessment of SpondyloArthritis international Society et al (2011) Ankylosing Spondylitis Disease Activity Score (ASDAS): defining cut-off values for disease activity states and improvement scores. Ann Rheum Dis 70:47–53
Kaltsonoudis E, Pelechas E, Voulgari PV, Drosos AA (2018) Unmet needs in the treatment of rheumatoid arthritis. An observational study and a real-life experience from a single university center. Semin Arthritis Rheum https://doi.org/10.1016/j.semarthrit.2018.06.003 (pii: S0049-0172(18)30188-4)
Baraliakos X, Kiltz U, Peters S et al (2017) Efficiency of treatment with non-steroidal anti-inflammatory drugs according to current recommendations in patients with radiographic and non-radiographic axial spondyloarthritis. Rheumatology 56:95–102
van der Heijde D, Kivitz A, Schiff MH, ATLAS Study Group et al (2006) Efficacy and safety of adalimumab in patients with ankylosing spondylitis: results of a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum 54:2136–2146
van der Heijde D, Dougados M, Landewé R et al (2017) Sustained efficacy, safety and patient-reported outcomes of certolizumab pegol in axial spondyloarthritis: 4-year outcomes from RAPID-axSpA. Rheumatology 56:1498–1509
Davis JC, van der Heijde DM, Braun J, Dougados M et al (2005) Sustained durability and tolerability of etanercept in ankylosing spondylitis for 96 weeks. Ann Rheum Dis 64:1557–1562
Inman RD, Davis JC Jr, Dv H et al (2008) Efficacy and safety of golimumab in patients with ankylosing spondylitis: results of a randomized, double-blind, placebo-controlled, phase III trial. Arthritis Rheum 58:3402–3412
Braun J, van der Horst-Bruinsma IE, Huang F et al (2011) Clinical efficacy and safety of etanercept versus sulfasalazine in patients with ankylosing spondylitis: a randomized, double-blind trial. Arthritis Rheum 63:1543–1551
Temekonidis TI, Alamanos Y, Nikas SN et al (2003) Infliximab therapy in patients with ankylosing spondylitis: an open label 12 month study. Ann Rheum Dis 62:1218–1220
Baraliakos X, Listing J, Fritz C et al (2011) Persistent clinical efficacy and safety of infliximab in ankylosing spondylitis after 8 years–early clinical response predicts long-term outcome. Rheumatology 50:1690–1699
Lambert RG, Salonen D, Rahman P et al (2007) Adalimumab significantly reduces both spinal and sacroiliac joint inflammation in patients with ankylosing spondylitis: a multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheum 56:4005–4014
Winthrop KL, Strand V, van der Heijde DM et al (2016) The unmet need in rheumatology: reports from the Targeted Therapies meeting 2016. Clin Exp Rheumatol 34(4 Suppl 98):69–76
Lubrano E, De Socio A, Perrotta FM (2017) Unmet Needs in Axial Spondyloarthritis. Clin Rev Allergy Immunol doi. https://doi.org/10.1007/s12016-017-8637-0
Giacomelli R, Afeltra A, Alunno A et al (2017) International consensus: What else can we do to improve diagnosis and therapeutic strategies in patients affected by autoimmune rheumatic diseases (rheumatoid arthritis, spondyloarthritides, systemic sclerosis, systemic lupus erythematosus, antiphospholipid syndrome and Sjogren’s syndrome)?: The unmet needs and the clinical grey zone in autoimmune disease management. Autoimmun Rev 16:911–924
Winthrop KL, Strand V, van der Heijde D et al (2018) The unmet need in rheumatology: reports from the targeted therapies meeting 2017. Clin Immunol 186:87–93
Baeten D, Sieper J, Braun J, MEASURE 1 Study Group, MEASURE 2 Study Group, et al (2015) Secukinumab, an interleukin-17A inhibitor, in ankylosing spondylitis. N Engl J Med 373:2534–2548
Funding
This study has no funding.
Author information
Authors and Affiliations
Contributions
EP and EK acquisition and analysis of the data. Manuscript drafting. PVV acquisition, analysis and interpretation of the data. AAD review of the manuscript and final approval.
Corresponding author
Ethics declarations
Conflict of interest
E. Pelechas declares no conflict of interest; Evripidis Kaltsonoudis declares no conflict of interest; Paraskevi V. Voulgari declares no conflict of interest; Alexandros A. Drosos declares no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pelechas, E., Kaltsonoudis, E., Voulgari, P.V. et al. Unmet needs in the treatment of ankylosing spondylitis: a long-term observational study from a single university center. Rheumatol Int 39, 663–668 (2019). https://doi.org/10.1007/s00296-019-04277-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-019-04277-w