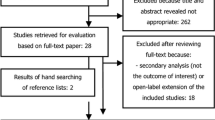
Abstract
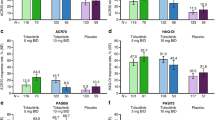
Psoriatic arthritis (PsA) is associated with progressive joint destruction and reduced quality of life. The time until a drug treatment starts to show an effect (TOA) is important for preventing joint destruction. The objective was to assess the time until onset of action of drugs when treating PsA. A systematic review of PsA drug trials was performed. Outcomes were: time until 25% of patients (TOA) reached (1) ≥ 20%, (2) ≥ 50% improvement in modified American College of Rheumatology response criteria (ACR), (3) ≥ 75% reduction in Psoriasis Area and Severity Index (PASI75). 95% confidence intervals were calculated extracting data from graphs using a novel method. Meta-analysis was conducted. Two head-to-head trials show no difference between ixekizumab and adalimumab or adalimumab and tofacitinib for TOA-ACR outcomes. For PASI75, ixekizumab had a faster onset than adalimumab. Infliximab plus MTX was faster than MTX alone. Pooled results from 32 study arms for TOA-ACR20 (week [95% CI]) are: < 2 weeks: infliximab (1.18 [0.72–1.65]), ixekizumab (1.04 [0.80–1.28]), tofacitinib (10 mg 1.56 [1.14–1.98]); ≤ 4 weeks: adalimumab (1.95 [1.35–2.55]), secukinumab (75 mg 1.89 [0.16–3.62], 150 mg 2.13 [1.34–2.91], 300 mg 2.26 [1.75–2.76]), tofacitinib (5 mg 2.20 [1.41–2.99]); 4 + weeks: apremilast, ustekinumab. For TOA-ACR50, all pooled point estimates are > 4 weeks. For TOA-PASI75, the range is between 2.24 [1.65–2.84] for ixekizumab and 6.03 [3.76–8.29] for adalimumab. Indirect, mixed comparison suggest a faster onset of infliximab, ixekizumab and tofacitinib compared to apremilast, methotrexate and ustekinumab for ACR20, not ACR50. For PASI75, ixekizumab is faster than adalimumab.



Similar content being viewed by others
Abbreviations
- ACR:
-
American College of Rheumatology
- ACR20:
-
≥ 20% improvement in modified American College of Rheumatology response criteria
- ACR50:
-
≥ 50% improvement in modified American College of Rheumatology response criteria
- ADA:
-
Adalimumab
- APR:
-
Apremilast
- bDMARD:
-
Biological disease-modifying anti-rheumatic drug
- BID:
-
Twice a day
- BIW:
-
Twice weekly
- BW:
-
Body weight
- CASPAR:
-
Classification criteria for the diagnosis of psoriatic arthritis
- CI:
-
Confidence intervals
- CRP:
-
C-reactive protein
- CSA:
-
Cyclosporine
- csDMARD:
-
Conventional synthetic disease-modifying anti-rheumatic drug
- CZP:
-
Certolizumab pegol
- d:
-
Day
- EE:
-
Early escape
- ES:
-
Point estimate
- ESR:
-
Erythrocyte sedimentation rate
- ETA:
-
Etanercept
- EULAR:
-
European League against Rheumatism
- GOL:
-
Golimumab
- GRAPPA:
-
Group for research and assessment of psoriasis and psoriatic arthritis
- GUS:
-
Guselkumab
- Hx:
-
History of
- ICTRP:
-
The International Clinical Trials Registry Platform
- INF:
-
Infliximab
- IQR:
-
Interquartile range
- iv:
-
Intravenous
- IXE:
-
Ixekizumab
- LE:
-
Lesion
- LEF:
-
Leflunomide
- LES:
-
Least squares
- MTX:
-
Methotrexate
- NA:
-
Not available
- NI:
-
No information
- NR:
-
Not restricted
- NSAID:
-
Non-steroidal anti-inflammatory drug
- PASI:
-
Psoriasis Area and Severity Index
- PASI75:
-
≥ 75% reduction in Psoriasis Area and Severity Index
- PBO:
-
Placebo
- PGA:
-
Physician’s global assessment
- PICOS:
-
Participants, interventions, comparisons, outcomes, study design
- PJC:
-
Painful joint count
- PRED:
-
Prednisolone
- PRISMA:
-
Preferred reporting items for systematic reviews and meta-analyses
- PsA:
-
Psoriatic arthritis
- PsARC:
-
Psoriatic arthritis response criteria
- Pso:
-
Cutaneous psoriasis
- pts:
-
Patients
- Q12W:
-
Every 12 weeks
- Q1W:
-
Once per week
- Q2W:
-
Every 2 weeks
- Q4W:
-
Every 4 weeks
- QD:
-
Once per day
- RA:
-
Rheumatoid arthritis
- RF:
-
Rheuma factor
- sc:
-
Subcutaneous
- SD:
-
Standard deviation
- SE:
-
Standard error
- SEC:
-
Secukinumab
- SJC:
-
Swollen joint count
- SSZ:
-
Sulfasalazine
- tbc:
-
Tuberculosis
- TJC:
-
Tender joint count
- TOF:
-
Tofacitinib
- tsDMARD:
-
Targeted synthetic disease-modifying anti-rheumatic drug
- TOA:
-
Time until onset of action
- TOA-ACR20:
-
Time until 25% of patients reach a ≥ 20% improvement in ACR criteria
- TOA-ACR50:
-
Time until 25% of patients reach a ≥ 50% improvement in ACR criteria
- TOA-PASI75:
-
Time until 25% of patients reach a ≥ 75% improvement in PASI
- UST:
-
Ustekinumab
- w:
-
Week/weeks
- y/yrs:
-
Year/years
References
Sankowski AJ, Łebkowska UM, Ćwikła J, Walecka I, Walecki J (2013) Psoriatic arthritis. Pol J Radiol 78:7–17. https://doi.org/10.12659/PJR.883763
Husted JA, Gladman DD, Farewell VT, Cook RJ (2001) Health-related quality of life of patients with psoriatic arthritis: a comparison with patients with rheumatoid arthritis. Arthritis Rheum 45:151–158. https://doi.org/10.1002/1529-0131(200104)45:2%3C151::aid-anr168%3E3.0.co;2-t
Gladman DD, Stafford-Brady F, Chang CH, Lewandowski K, Russell ML (1990) Longitudinal study of clinical and radiological progression in psoriatic arthritis. J Rheumatol 17:809–812
Eder L, Gladman DD (2013) Psoriatic arthritis: phenotypic variance and nosology. Curr Rheumatol Rep 15:316. https://doi.org/10.1007/s11926-013-0316-4
Husni ME, Merola JF, Davin S (2017) The psychosocial burden of psoriatic arthritis. Semin Arthritis Rheum 47:351–360. https://doi.org/10.1016/j.semarthrit.2017.05.010
Ogdie A, Weiss P (2015) The epidemiology of psoriatic arthritis. Rheum Dis Clin N Am 41:545–568
Goulabchand R, Mouterde G, Barnetche T, Lukas C, Morel J, Combe B (2014) Effect of tumour necrosis factor blockers on radiographic progression of psoriatic arthritis: a systematic review and meta-analysis of randomised controlled trials. Ann Rheum Dis 73:414–419. https://doi.org/10.1136/annrheumdis-2012-202641
Gossec L, Smolen JS, Ramiro S, de Wit M, Cutolo M, Dougados M, Emery P, Landewé R, Oliver S, Aletaha D, Betteridge N, Braun J, Burmester G, Cañete JD, Damjanov N, FitzGerald O, Haglund E, Helliwell P, Kvien TK, Lories R, Luger T, Maccarone M, Marzo-Ortega H, McGonagle D, McInnes IB, Olivieri I, Pavelka K, Schett G, Sieper J, van den Bosch F, Veale DJ, Wollenhaupt J, Zink A, van der Heijde D (2015) European League Against Rheumatism (EULAR) recommendations for the management of psoriatic arthritis with pharmacological therapies: 2015 update. Ann Rheum Dis. https://doi.org/10.1136/annrheumdis-2015-208337
Smolen JS, van der Heijde D, Machold KP, Aletaha D, Landewe R (2014) Proposal for a new nomenclature of disease-modifying antirheumatic drugs. Ann Rheum Dis 73:3–5. https://doi.org/10.1136/annrheumdis-2013-204317
Sabaté E (2003) Adherence to long-term therapies: evidence for action. World HealthOrganization. https://www.who.int/chp/knowledge/publications/adherence_report/en/. Accessed 12 Jan 2017
Higgins JPT, Green S (2011) Cochrane handbook for systematic reviews of interventions version 5.1.0. The Cochrane Collaboration. http://handbook.cochrane.org. Accessed 12 Jan 2017
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, Clarke M, Devereaux PJ, Kleijnen J, Moher D (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 339:b2700. https://doi.org/10.1136/bmj.b2700
Gladman DD, Mease PJ, Strand V, Healy P, Helliwell PS, Fitzgerald O, Gottlieb AB, Krueger GG, Nash P, Ritchlin CT, Taylor W, Adebajo A, Braun J, Cauli A, Carneiro S, Choy E, Dijkmans B, Espinoza L, van der Heijde D, Husni E, Lubrano E, McGonagle D, Qureshi A, Soriano ER, Zochling J (2007) Consensus on a core set of domains for psoriatic arthritis. J Rheumatol 34:1167–117014
Nast A, Sporbeck B, Rosumeck S, Pathirana D, Jacobs A, Werner RN, Schmitt J (2013) Which antipsoriatic drug has the fastest onset of action? Systematic review on the rapidity of the onset of action. J Investig Dermatol 133:1963–1970. https://doi.org/10.1038/jid.2013.78
Dean AGSK, Soe MM (2013) OpenEpi: open source epidemiologic statistics for public health, version. https://openepi.com. Accessed 06 Jun 2018
Higgins JPT, Sterne JAC, Savović J, Page MJ, Hróbjartsson A, Boutron I, Reeves B, Eldridge S (2016) A revised tool for assessing risk of bias in randomized trials. Cochrane Database Syst Rev 10:29–31. https://doi.org/10.1002/14651858.CD201601
Deeks JJ, Higgins JPT, Altman DG (2008) Analysing data and undertaking meta-analyses. Cochrane Handb Syst Rev Interv. https://doi.org/10.1002/9780470712184.ch9
Greenland S, Senn SJ, Rothman KJ, Carlin JB, Poole C, Goodman SN, Altman DG (2016) Statistical tests, P values, confidence intervals, and power: a guide to misinterpretations. Eur J Epidemiol 31:337–350. https://doi.org/10.1007/s10654-016-0149-3
Baranauskaite A, Raffayova H, Kungurov NV, Kubanova A, Venalis A, Helmle L, Srinivasan S, Nasonov E, Vastesaeger N, Investigators R (2012) Infliximab plus methotrexate is superior to methotrexate alone in the treatment of psoriatic arthritis in methotrexate-naive patients: the RESPOND study. Ann Rheum Dis 71:541–548
Mease PJ, Hall S, Gerald OF, Heijde D, Merola JF, Avila-Zapata F, Cieslak D, Graham D, Wang C, Menon S, Hendrikx T, Kanik K (2017) Efficacy and safety of tofacitinib, an oral janus kinase inhibitor, or adalimumab in patients with active psoriatic arthritis and an inadequate response to conventional synthetic DMARDS: a randomized, placebo-controlled, phase 3 trial. Arthritis Rheumatol 68:3975–3978. https://doi.org/10.1002/art.39977
Mease PJ, van der Heijde D, Ritchlin CT, Cuchacovich RS, Shuler CL, Lin CY et al (2016) Ixekizumab, an interleukin-17A specific monoclonal antibody, for the treatment of biologic-naive patients with active psoriatic arthritis: results from the 24-week randomised, double-blind, placebocontrolled and active (adalimumab)-controlled period of the phase III trial SPIRIT-P1. Ann Rheum Dis 76:79–87. https://doi.org/10.1136/annrheumdis-2016-209709
Schett G, Wollenhaupt J, Papp K, Joos R, Rodrigues JF, Vessey AR, Hu C, Stevens R, de Vlam KL (2012) Oral apremilast in the treatment of active psoriatic arthritis: results of a multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheum 64:3156–3167
Mease PJ, Fleischmann R, Deodhar AA, Wollenhaupt J, Khraishi M, Kielar D, Woltering F, Stach C, Hoepken B, Arledge T, van der Heijde D (2014) Effect of certolizumab pegol on signs and symptoms in patients with psoriatic arthritis: 24-week results of a Phase 3 double-blind randomised placebo-controlled study (RAPID-PsA). Ann Rheum Dis 73:48–55
Kavanaugh A, van der Heijde D, McInnes IB, Mease P, Krueger GG, Gladman DD, Gomez-Reino J, Papp K, Baratelle A, Xu W, Mudivarthy S, Mack M, Rahman MU, Xu Z, Zrubek J, Beutler A (2012) Golimumab in psoriatic arthritis: 1-year clinical efficacy, radiographic, and safety results from a phase III, randomized, placebo-controlled trial. Arthritis Rheum 64:2504–2517
Kavanaugh A, McInnes I, Mease P, Krueger GG, Gladman D, Gomez-Reino J, Papp K, Zrubek J, Mudivarthy S, Mack M, Visvanathan S, Beutler A (2009) Golimumab, a new human tumor necrosis factor alpha antibody, administered every four weeks as a subcutaneous injection in psoriatic arthritis: twenty-four-week efficacy and safety results of a randomized, placebo-controlled study. Arthritis Rheum 60:976–986
Mease PJ, McInnes IB, Kirkham B, Kavanaugh A, Rahman P, van der Heijde D, Landewe R, Nash P, Pricop L, Yuan J, Richards HB, Mpofu S, Future Study Group (2015) Secukinumab inhibition of interleukin-17A in patients with psoriatic arthritis. N Engl J Med 373:1329–1339
McInnes IB, Mease PJ, Kirkham B, Kavanaugh A, Ritchlin CT, Rahman P, Heijde D, Landewé R, Conaghan PG, Gottlieb AB, Richards H, Pricop L, Ligozio G, Patekar M, Mpofu S (2015) Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 386:1137–1146. https://doi.org/10.1016/S0140-6736(15)61134-5
Nash P, Mease PJ, McInnes IB, Rahman P, Ritchlin CT, Blanco R, Dokoupilova E, Andersson M, Kajekar R, Mpofu S, Pricop L (2018) Efficacy and safety of secukinumab administration by autoinjector in patients with psoriatic arthritis: results from a randomized, placebo-controlled trial (FUTURE 3). Arthritis Res Ther 20:47. https://doi.org/10.1186/s13075-018-1551-x
Gladman DD, Rigby W, Azevedo VF, Behrens F, Blanco R, Kaszuba A, Kudlacz E, Wang C, Menon S, Hendrikx T, Kanik KS (2017) Efficacy and safety of tofacitinib, an oral janus kinase inhibitor, in patients with active psoriatic arthritis and an inadequate response to tumor necrosis factor inhibitors: opal beyond, a randomized, double blind, placebo-controlled, phase 3 trial. Arthritis Rheumatol 68:4371–4375. https://doi.org/10.1002/art.39977
Asahina A, Etoh T, Igarashi A, Imafuku S, Saeki H, Shibasaki Y, Tomochika Y, Toyoizumi S, Nagaoka M, Ohtsuki M (2016) Oral tofacitinib efficacy, safety and tolerability in Japanese patients with moderate to severe plaque psoriasis and psoriatic arthritis: a randomized, double-blind, phase 3 study. J Dermatol 43:869–880
McInnes IB, Kavanaugh A, Gottlieb AB, Puig L, Rahman P, Ritchlin C, Brodmerkel C, Li S, Wang Y, Mendelsohn AM, Doyle MK, Psummit Study Group (2013) Efficacy and safety of ustekinumab in patients with active psoriatic arthritis: 1 year results of the phase 3, multicentre, double-blind, placebo-controlled PSUMMIT 1 trial. Lancet 382:780–789
Ritchlin C, Rahman P, Kavanaugh A, McInnes IB, Puig L, Li S, Wang Y, Shen YK, Doyle MK, Mendelsohn AM, Gottlieb AB, Psummit Study Group (2014) Efficacy and safety of the anti-IL-12/23 p40 monoclonal antibody, ustekinumab, in patients with active psoriatic arthritis despite conventional non-biological and biological anti-tumour necrosis factor therapy: 6-month and 1-year results of the phase 3, multicentre, double-blind, placebo-controlled, randomised PSUMMIT 2 trial. Ann Rheum Dis 73:990–999
Mease P, Deodhar A, Fleischmann R, Wollenhaupt J, Gladman D, Leszczynski P, Vitek P, Turkiewicz A, Khraishi M, FitzGerald O, Landewe R, de Longueville M, Hoepken B, Peterson L, van der Heijde D (2015) Effect of certolizumab pegol over 96 weeks in patients with psoriatic arthritis with and without prior antitumour necrosis factor exposure. RMD Open 1:e000119. https://doi.org/10.1136/rmdopen-2015-000119
Nash P, Kirkham B, Okada M, Rahman P, Combe B, Burmester G-R, Adams D, Kerr L, Lee C, Shuler C, Genovese M (2017) Ixekizumab for the treatment of patients with active psoriatic arthritis and an inadequate response to tumour necrosis factor inhibitors: results from the 24-week randomised, double-blind, placebo-controlled period of the SPIRIT-P2 phase 3 trial. Lancet 389:2317–2327. https://doi.org/10.1016/S0140-6736%2817%2931429-0
Antoni C, Krueger GG, de Vlam K, Birbara C, Beutler A, Guzzo C, Zhou B, Dooley LT, Kavanaugh A (2005) Infliximab improves signs and symptoms of psoriatic arthritis: results of the IMPACT 2 trial. Ann Rheum Dis 64:1150–1157. https://doi.org/10.1136/ard.2004.032268
Antoni CE, Kavanaugh A, Kirkham B, Tutuncu Z, Burmester GR, Schneider U, Furst DE, Molitor J, Keystone E, Gladman D, Manger B, Wassenberg S, Weier R, Wallace DJ, Weisman MH, Kalden JR, Smolen J (2005) Sustained benefits of infliximab therapy for dermatologic and articular manifestations of psoriatic arthritis: results from the infliximab multinational psoriatic arthritis controlled trial (IMPACT). Arthritis Rheum 52:1227–1236
Genovese MC, Mease PJ, Thomson GT, Kivitz AJ, Perdok RJ, Weinberg MA, Medich J, Sasso EH (2007) Safety and efficacy of adalimumab in treatment of patients with psoriatic arthritis who had failed disease modifying antirheumatic drug therapy. J Rheumatol 34:1040–1050
Gottlieb A, Menter A, Mendelsohn A, Shen YK, Li S, Guzzo C, Fretzin S, Kunynetz R, Kavanaugh A (2009) Ustekinumab, a human interleukin 12/23 monoclonal antibody, for psoriatic arthritis: randomised, double-blind, placebo-controlled, crossover trial. Lancet 373:633–640. https://doi.org/10.1016/S0140-6736(09)60140-9
Kavanaugh A, Husni ME, Harrison DD, Kim L, Lo KH, Leu JH, Hsia EC (2017) Safety and efficacy of intravenous golimumab in patients with active psoriatic arthritis: results through week twenty-four of the GO-VIBRANT study. Arthritis Rheumatol 69:2151–2161. https://doi.org/10.1002/art.40226
Mease P, Hall S, FitzGerald O, van der Heijde D, Merola JF, Avila-Zapata F, Cieślak D, Graham D, Wang C, Menon S, Hendrikx T, Kanik KS (2017) Tofacitinib or adalimumab versus placebo for psoriatic arthritis. N Engl J Med 377:1537–1550. https://doi.org/10.1056/NEJMoa161597542
McInnes IB, Sieper J, Braun J, Emery P, van der Heijde D, Isaacs JD, Dahmen G, Wollenhaupt J, Schulze-Koops H, Kogan J, Ma S, Schumacher MM, Bertolino AP, Hueber W, Tak PP (2014) Efficacy and safety of secukinumab, a fully human anti-interleukin-17A monoclonal antibody, in patients with moderate-to-severe psoriatic arthritis: a 24-week, randomised, double-blind, placebo-controlled, phase II proof-of-concept trial. Ann Rheum Dis 73:349–356
Mease PJ, Gladman DD, Ritchlin CT, Ruderman EM, Steinfeld SD, Choy EH, Sharp JT, Ory PA, Perdok RJ, Weinberg MA, Adalimumab Effectiveness in Psoriatic Arthritis Trial Study Group (2005) Adalimumab for the treatment of patients with moderately to severely active psoriatic arthritis: results of a double-blind, randomized, placebo-controlled trial. Arthritis Rheum 52:3279–3289. https://doi.org/10.1002/art.21306
Mease PJ, Kivitz AJ, Burch FX, Siegel EL, Cohen SB, Ory P, Salonen D, Rubenstein J, Sharp JT, Tsuji W (2004) Etanercept treatment of psoriatic arthritis: safety, efficacy, and effect on disease progression. Arthritis Rheum 50(7):2264–2272
Torii H, Nakagawa H, Japanese Infliximab Study investigators (2010) Infliximab monotherapy in Japanese patients with moderate-to-severe plaque psoriasis and psoriatic arthritis. A randomized, double-blind, placebo-controlled multicenter trial. J Dermatol Sci 59:40–49. https://doi.org/10.1016/j.jdermsci.2010.04.014
van Kuijk AW, Gerlag DM, Vos K, Wolbink G, de Groot M, de Rie MA, Zwinderman AH, Dijkmans BA, Tak PP (2009) A prospective, randomised, placebo-controlled study to identify biomarkers associated with active treatment in psoriatic arthritis: effects of adalimumab treatment on synovial tissue. Ann Rheum Dis 68:1303–1309. https://doi.org/10.1136/ard.2008.09138947
Mease PJ, Genovese MC, Weinblatt M, Peloso PM, Chen K, Li Y, Mansikka HT, Khatri A, Othman AA, Wishart N, Liu J, Padley RJ (2017) Safety and efficacy of ABT-122, a TNF and IL-17-targeted dual variable domain (DVD)-IgTM, in psoriatic arthritis patients with inadequate response to methotrexate: results from a phase 2 trial. Arthritis Rheumatol 68:1268–1270. https://doi.org/10.1002/art.39977
Gladman D, Rigby W, Azevedo VF, Behrens F, Blanco R, Kaszuba A, Kudlacz E, Wang C, Menon S, Hendrikx T, Kanik KS (2017) Tofacitinib for psoriatic arthritis in patients with an inadequate response to TNF inhibitors. N Engl J Med 377:1525–1536. https://doi.org/10.1056/NEJMoa1615977
Nash P, Ohson K, Walsh J, Delev N, Nguyen D, Teng L, Gómez-Reino JJ, Aelion JA (2018) Early and sustained efficacy with apremilast monotherapy in biological-naive patients with psoriatic arthritis: a phase IIIB, randomised controlled trial (ACTIVE). Ann Rheum Dis 77:690–698. https://doi.org/10.1136/annrheumdis-2017-211568
Song F, Hooper L, Loke Y (2013) Publication bias: what is it? How do we measure it? How do we avoid it? Open Access J Clin Trials 5:71–81. https://doi.org/10.2147/OAJCT.S34419
Orbai A-M, de Wit M, Mease P, Shea JA, Gossec L, Leung YY, Tillett W, Elmamoun M, Callis Duffin K, Campbell W, Christensen R, Coates L, Dures E, Eder L, FitzGerald O, Gladman D, Goel N, Grieb SD, Hewlett S, Hoejgaard P, Kalyoncu U, Lindsay C, McHugh N, Shea B, Steinkoenig I, Strand V, Ogdie A (2016) International patient and physician consensus on a psoriatic arthritis core outcome set for clinical trials. Ann Rheum Dis. https://doi.org/10.1136/annrheumdis-2016-210242
Papp KA, Lebwohl MG (2017) Onset of action of biologics in patients with moderate-to-severe psoriasis. J Drugs Dermatol 17:247–250
Savage LJ, Wittmann M, McGonagle D, Helliwell PS (2015) Ustekinumab in the treatment of psoriasis and psoriatic arthritis. Rheumatol Ther 2:1–16. https://doi.org/10.1007/s40744-015-0010-2
Kingsley GH, Kowalczyk A, Taylor H, Ibrahim F, Packham JC, McHugh NJ, Mulherin DM, Kitas GD, Chakravarty K, Tom BD, O’Keeffe AG, Maddison PJ, Scott DL (2012) A randomized placebo-controlled trial of methotrexate in psoriatic arthritis. Rheumatology 51:1368–1377. https://doi.org/10.1093/rheumatology/kes001
Jones G, Crotty M, Brooks P (2000) Interventions for treating psoriatic arthritis. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD000212
Higgins JP, Thompson SG, Spiegelhalter DJ (2008) A re-evaluation of random-effects meta-analysis. J R Stat Soc Ser A (Statistics in Society) 172:137–159. https://doi.org/10.1111/j.1467-985X.2008.00552.x
Kavanaugh A, Menter A, Mendelsohn A, Shen YK, Lee S, Gottlieb AB (2010) Effect of ustekinumab on physical function and health-related quality of life in patients with psoriatic arthritis: a randomized, placebo-controlled, phase II trial. Curr Med Res Opin 26(10):2385–2392. https://doi.org/10.1185/03007995.2010.515804
Funding
The project was funded by Eli Lilly Germany. The funder had no role in the design, conduct, writing and editing of the project.
Author information
Authors and Affiliations
Contributions
PAP: design, data acquisition, analysis and interpretation, and drafting the manuscript. CD: design, data acquisition, analysis and interpretation. LE: design, data acquisition. AN: conception, design. RNW: conception, design and data interpretation. All authors have revised the manuscript critically for important intellectual content and approved the final manuscript. Furthermore, all authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. No external writing or editing support was involved.
Corresponding author
Ethics declarations
Conflict of interest
Authors Phuong Anh Pham (PAP), Dr. Corinna Dressler (CD), Ricardo N. Werner, MD (RNW) declare that they have no conflicts of interest. Author Lisa Eisert, MD (LE) has received seminar participation fees from Pfizer (Enbrel), Leo Pharma (Daivobet, Protopic, Enstilar). Author Prof. Alexander Nast, MD (AN) has received institutional research grants/participated as an investigator (without personal honoraria) in research projects, advisory activities or trials from the following companies with an interest in psoriatic arthritis: Lilly, Novartis, Dermira. AN has received personal honoraria for lectures/educational activities from the following companies which—to his knowledge—currently have no interest in psoriatic arthritis: Bayer Healthcare, Pierre Fabre, Boehringer Ingelheim.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pham, P.A., Dressler, C., Eisert, L. et al. Time until onset of action when treating psoriatic arthritis: meta-analysis and novel approach of generating confidence intervals. Rheumatol Int 39, 605–618 (2019). https://doi.org/10.1007/s00296-019-04244-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-019-04244-5