Abstract
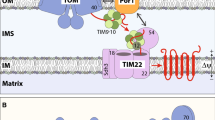
Mitochondrial biogenesis and functions rely on transport of their resident proteins as well as small molecules/ions across their membranes. The TOM complex functions as a protein entry gate for most mitochondrial proteins and mitochondrial porin facilitates transport of small-molecule metabolites and ions. We recently found a novel role of porin in regulation of the TOM complex assembly, the dynamic exchange between the dimer and trimer, and different substrate specificities of the dimer and trimer. Using distinct assembly forms customized for different client proteins, the TOM complex can handle ~ 1000 different mitochondrial protein for their import into mitochondria.

Similar content being viewed by others
References
Ellenrieder L, Dieterle MP, Doan KN, Mårtensson CU, Floerchinger A, Campo ML, Pfanner N, Becker T (2019) Dual role of mitochondrial Porin in metabolite transport across the outer membrane and protein transfer to the inner membrane. Mol Cell 73:1056–1065
Gornicka A, Bragoszewski P, Chroscicki P, Wenz L-S, Schultz C, Rehling P, Chacinska A (2014) A discrete pathway for the transfer of intermembrane space proteins across the outer membrane of mitochondria. Mol Biol Cell 25:3999–4009
Model K, Meisinger C, Prinz T, Wiedemann N, Truscott KN, Pfanner N, Ryan MT (2001) Multistep assembly of the protein import channel of the mitochondrial outer membrane. Nat Struct Biol 8:361–370
Sakaue H, Shiota T, Ishizaka N, Kawano S, Tamura Y, Tan KS, Imai K, Motono C, Hirokawa T, Taki K, Miyata N, Kuge O, Lithgow T, Endo T (2019) Porin associates with Tom22 to regulate the mitochondrial protein gate assembly. Mol Cell 73:1044–1055
Shiota T, Imai K, Qiu J, Hewitt VL, Tan K, Shen H-H, Sakiyama N, Fukasawa Y, Hayat S, Kamiya M, Elofsson A, Tomii K, Horton P, Wiedemann N, Pfanner N, Lithgow T, Endo T (2015) Molecular architecture of the active mitochondrial protein gate. Science 349:1544–1548
Yamano K, Tanaka-Yamano S, Endo T (2010) Mdm10 as a dynamic constituent of the TOB/SAM complex directs coordinated assembly of Tom40. EMBO Rep 11:187–193
Acknowledgements
This work was supported by JSPS KAKENHI to T.E. (15H05705 and 2222703) and a JST CREST grant to T.E. (JPMJCR12M1).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Kupiec.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sakaue, H., Endo, T. Regulation of the protein entry gate assembly by mitochondrial porin. Curr Genet 65, 1161–1163 (2019). https://doi.org/10.1007/s00294-019-00979-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00294-019-00979-7