Abstract
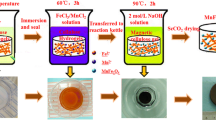
A magnetic composite microsphere, calcium alginate (CA)/carboxymethyl cellulose (CMC)@MnFe2O4, was prepared using CA and CMC encapsulated MnFe2O4 through blending and cross-linking for the removal of Cu(II) from aqueous solution. The CA/CMC@MnFe2O4 was characterized in detail by Fourier transform infrared spectroscopy, X-ray diffraction, scanning electron microscopy, and X-ray phosphorescence spectroscopy. In addition, various adsorption conditions including the initial pH of Cu(II) solution, equilibrium contact time, and initial Cu(II) concentration were tested. The adsorption data fit better to the Langmuir isotherm and follow the pseudo-second-order model, suggesting that it is a monolayer adsorption process with chemisorption as the rate-limiting step. The maximum adsorption capacity of the CA/CMC@MnFe2O4 was 77.22 mg g−1 at 25 ℃ and pH 5. Cu(II) removal mechanisms of CA/CMC@MnFe2O4 follow ion exchange as well as the formation of complexes as evidenced based on the microstructure analysis and adsorption data fitting.










Similar content being viewed by others
References
Guan X, Yang H, Sun Y, Qiao J (2019) Enhanced immobilization of chromium(VI) in soil using sulfidated zero-valent iron. Chemosphere 228:370–376
Lachowicz JI, Delpiano GR, Zanda D, Piludu M, Sanjust E, Monduzzi M, Salis A (2019) Adsorption of Cu2+ and Zn2+ on SBA-15 mesoporous silica functionalized with triethylenetetramine chelating agent. J Environ Chem Eng 532:474–484
Sun J, Chen Y, Yu H, Yan L, Du B, Pei Z (2018) Removal of Cu2+, Cd2+ and Pb2+ from aqueous solutions by magnetic alginate microsphere based on Fe3O4/MgAl-layered double hydroxide. J Colloid Interface Sci 532:474–484
Yu C, Wang M, Dong X, Shi Z, Zhang X, Lin Q (2017) Removal of Cu(II) from aqueous solution using Fe3O4–alginate modified biochar microspheres. RSC Adv 7:53135–53144
Asadi R, Abdollahi H, Gharabaghi M, Boroumand Z (2020) Effective removal of Zn(II) ions from aqueous solution by the magnetic MnFe2O4 and CoFe2O4 spinel ferrite nanoparticles with focuses on synthesis, characterization, adsorption, and desorption. Adv Powder Technol 31:1480–1489
Ren Y, Li N, Feng J, Luan T, Wen Q, Li Z, Zhang M (2012) Adsorption of Pb(II) and Cu(II) from aqueous solution on magnetic porous ferrospinel MnFe2O4. J Colloid Interface Sci 367:415–421
Giakisikli G, Anthemidis AN (2013) Magnetic materials as sorbents for metal/metalloid preconcentration and/or separation. A review. Anal Chim Acta 789:1–16
Zheng XM, Dou JF, Xia M, Ding AZ (2017) Ammonium-pillared montmorillonite-CoFe2O4 composite caged in calcium alginate beads for the removal of Cs+ from wastewater. Carbohydr Polym 167:306–316
Talbot D, Abramson S, Griffete N, Bée A (2018) pH-sensitive magnetic alginate/γ-Fe2O3 nanoparticles for adsorption/desorption of a cationic dye from water. J Water Process Eng 25:301–308
J. R, J. J, (2019) Simultaneous removal of binary dye from textile effluent using cobalt ferrite-alginate nanocomposite: Performance and mechanism. Microchem J 145:791–800
Ren H, Gao Z, Wu D, Jiang J, Sun Y, Luo C (2016) Efficient Pb(II) removal using sodium alginate-carboxymethyl cellulose gel beads: Preparation, characterization, and adsorption mechanism. Carbohydr Polym 137:402–409
Vijayalakshmi K, Devi BM, Latha S, Gomathi T, Sudha PN, Venkatesan J, Anil S (2017) Batch adsorption and desorption studies on the removal of lead(II) from aqueous solution using nanochitosan/sodium alginate/microcrystalline cellulose beads. Int J Biol Macromol 104:1483–1494
Taleb MFA (2014) Adsorption and photocatalytic degradation of 2-CP in wastewater onto CS/CoFe2O4 nanocomposite synthesized using gamma radiation. Carbohydr Polym 114:65–72
Hu Q, Liu Y, Gu X, Zhao Y (2017) Adsorption behavior and mechanism of different arsenic species on mesoporous MnFe2O4 magnetic nanoparticles. Chemosphere 181:328–336
Dewangan T, Tiwari A, Bajpai AK (2011) Removal of chromium(VI) ions by adsorption onto binary biopolymeric beads of sodium alginate and carboxymethyl cellulose. J Dispers Sci Technol 32:1075–1082
Wu T, Mao L, Wang H (2017) Adsorption of fluoride from aqueous solution by using hybrid adsorbent fabricated with Mg/Fe composite oxide and alginate via a facile method. J Fluorine Chem 200:8–17
Guo Y, Tang W, Wu J, Huang Z, Dai J (2014) Mechanism of Cu(II) adsorption inhibition on biochar by its aging process. J Environ Sci 26:2123–2130
Verma R, Asthana A, Singh AK, Prasad S, Susan MABH (2017) Novel glycine-functionalized magnetic nanoparticles entrapped calcium alginate beads for effective removal of lead. Microchem J 130:168–178
Li X, Qi Y, Li Y, Zhang Y, He X, Wang Y (2013) Novel magnetic beads based on sodium alginate gel crosslinked by zirconium(IV) and their effective removal for Pb2+ in aqueous solutions by using a batch and continuous systems. Bioresour Technol 142:611–619
Xiong P, Hu C, Fan Y, Zhang W, Zhu J, Wang X (2014) Ternary manganese ferrite/graphene/polyaniline nanostructure with enhanced electrochemical capacitance performance. J Power Sources 266:384–392
Liu Y, Fu R, Sun Y, Zhou X, Baig SA, Xu X (2016) Multifunctional nanocomposites Fe3 O4@SiO2-EDTA for Pb(II) and Cu(II) removal from aqueous solutions. Appl Surf Sci 369:267–276
Hu X, Long L, Gong T, Zhang J, Yan J, Xue Y (2020) Enhanced alginate-based microsphere with the pore-forming agent for efficient removal of Cu2+. Chemosphere 240:124860
Jin H, Capareda S, Chang Z, Gao J, Xu Y, Zhang J (2014) Biochar pyrolytically produced from municipal solid wastes for aqueous As(V) removal: adsorption property and its improvement with KOH activation. Bioresour Technol 169:622–629
Liu C, Ye J, Lin Y, Wu J, Price GW, Burton D, Wang Y (2020) Removal of cadmium(II) using water hyacinth (Eichhornia crassipes) biochar alginate beads in aqueous solutions. Environ Pollut 264:114785
Zeng H, Yu Y, Wang F, Zhang J, Li D (2020) Arsenic(V) removal by granular adsorbents made from water treatment residuals materials and chitosan. Colloids Surf A 585:124036
Zhao LX, Liang JL, Li N, Xiao H, Chen LZ, Zhao RS (2020) Kinetic, thermodynamic and isotherm investigations of Cu2+ and Zn2+ adsorption on LiAl hydrotalcite-like compound. Sci Total Environ 716:137120
Kameda T, Honda R, Kumagai S, Saito Y, Yoshioka T (2019) Adsorption of Cu2+ and Ni2+ by tripolyphosphate-crosslinked chitosan-modified montmorillonite. J Solid State Chem 277:143–148
Karami S, Zeynizadeh B (2019) Reduction of 4-nitrophenol by a disused adsorbent: EDA-functionalized magnetic cellulose nanocomposite after the removal of Cu2+. Carbohydr Polym 211:298–307
Zhang Z, He S, Zhang Y, Zhang K, Wang J, Jing R, Yang X, Hu Z, Lin X, Li Y (2019) Spectroscopic investigation of Cu2+, Pb2+ and Cd2+ adsorption behaviors by chitosan-coated argillaceous limestone: Competition and mechanisms. Environ Pollut 254:112938
Ojemaye MO, Okoh OO, Okoh AI (2017) Adsorption of Cu2+ from aqueous solution by a novel material; azomethine functionalized magnetic nanoparticles. Sep Purif Technol 183:204–215
Mokadem Z, Mekki S, Saïdi-Besbes S, Agusti G, Elaissari A, Derdour A (2017) Triazole containing magnetic core-silica shell nanoparticles for Pb2+, Cu2+ and Zn2+ removal. Arab J Chem 10:1039–1051
Hu C, Li G, Wang Y, Li F, Guo G, Hu H (2017) The effect of pH on the bonding of Cu2+ and chitosan-montmorillonite composite. Int J Biol Macromol 103:751–757
Guo Y, Jia Z, Wang S, Su Y, Ma H, Wang L, Meng W (2019) Sandwich membranes based on PVDF-g-4VP and surface modified graphene oxide for Cu(II) adsorption. J Hazard Mater 377:17–23
Bediako JK, Lin S, Sarkar AK, Zhao Y, Choi J-W, Song M-H, Wei W, Reddy DHK, Cho C-W, Yun Y-S (2020) Benignly-fabricated crosslinked polyethylenimine/calcium-alginate fibers as high-performance adsorbents for effective recovery of gold. J Clean Prod 252:119389
Gao X, Guo C, Hao J, Zhao Z, Long H, Li M (2020) Selective adsorption of Pd (II) by ion-imprinted porous alginate beads: experimental and density functional theory study. Int J Biol Macromol 157:401–413
Liang HF, Wang ZC (2010) Adsorption of bovine serum albumin on functionalized silica-coated magnetic MnFe2O4 nanoparticles. Mater Chem Phys 124:964–969
Xu W, Song Y, Dai K, Sun S, Liu G, Yao J (2018) Novel ternary nanohybrids of tetraethylenepentamine and graphene oxide decorated with MnFe2O4 magnetic nanoparticles for the adsorption of Pb(II). J Hazard Mater 358:337–345
Zhou YT, Branford-White C, Nie HL, Zhu LM (2009) Adsorption mechanism of Cu2+ from aqueous solution by chitosan-coated magnetic nanoparticles modified with alpha-ketoglutaric acid. Colloids Surf B Biointerfaces 74:244–252
Mahdavinia GR, Mousanezhad S, Hosseinzadeh H, Darvishi F, Sabzi M (2016) Magnetic hydrogel beads based on PVA/sodium alginate/laponite RD and studying their BSA adsorption. Carbohydr Polym 147:379–391
Roh H, Yu M-R, Yakkala K, Koduru JR, Yang J-K, Chang Y-Y (2015) Removal studies of Cd(II) and explosive compounds using buffalo weed biochar-alginate beads. J Ind Eng Chem 26:226–233
Huang S, Jiang S, Pang H, Wen T, Asiri AM, Alamry KA, Alsaedi A, Wang X, Wang S (2019) Dual functional nanocomposites of magnetic MnFe2O4 and fluorescent carbon dots for efficient U(VI) removal. Chem Eng J 368:941–950
Verma M, Kumar A, Singh KP, Kumar R, Kumar V, Srivastava CM, Rawat V, Rao G, Kumari S, Sharma P, Kim H (2020) Graphene oxide-manganese ferrite (GO-MnFe2O4) nanocomposite: one-pot hydrothermal synthesis and its use for adsorptive removal of Pb2+ ions from aqueous medium. J Mol Liq 315:113769
Acknowledgements
This study was supported by the natural Science Foundation of Hainan Province of China (Project No. 219QN208); the Key Research and Development Project of Hainan Province of China (Project No. ZDYF 2020079), the Education Department of Hainan Province (Project No. Hnky2019-33).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yu, C., Li, H., Ma, H. et al. Characteristics and mechanism of Cu(II) adsorption on prepared calcium alginate/carboxymethyl cellulose@MnFe2O4. Polym. Bull. 79, 1201–1216 (2022). https://doi.org/10.1007/s00289-021-03555-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-021-03555-7