Abstract
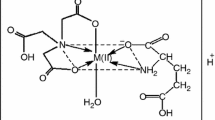
The binding of zinc(II) ions onto γ-pga was studied in aqueous solution as a function of contact time, solution pH, initial concentration of metal ion, and solution temperature, respectively. IR and 13C NMR spectra of γ-pga and Zn(γ-pga) complex revealed binding of zinc(II) with carboxylate and amide groups on γ-pga. The strong interaction between zinc(II) ions and γ-pga occurred at pH 5. The binding kinetic data followed a pseudo-second-order kinetic model. The equilibrium binding data were fitted well by Langmuir isotherm model. The maximum amount of zinc(II) ions bound to γ-pga was estimated to be 13.97 mmol/g at 30 °C and 20.58 mmol/g at 45 °C. The values of activation energy (Ea 18.23 kJ/mol), changes in free energy (∆G°), enthalpy (∆H°), and entropy (∆S°) indicate that the formation of Zn(γ-pga) complex is an endothermic spontaneous physisorption process. The antimicrobial activity of Zn(γ-pga) complex was examined against a gram-negative bacteria (i.e., Escherichia coli) and a gram-positive bacteria (i.e., Bacillus subtilis). The IC50 values of Zn(γ-pga) complex were estimated to be (0.76 ± 0.03) mmol/L for E. coli and (0.94 ± 0.02) mmol/L for B. subtilis, respectively. Therefore, Zn(γ-pga) complex can be used as an antimicrobial agent against gram-positive and gram-negative microorganisms.











Similar content being viewed by others
References
Xu S, Zhang R, Zhao W, Zhu Y, Wei W, Liu X, Luo J (2017) Self-assembled polymeric nanoparticles film stabilizing gold nanoparticles as a versatile platform for ultrasensitive detection of carcino-embryonic antigen. Biosens Bioelectron 92:570–576
Luo Z, Yuan G, Liu J, Qiu H, Zhao M, Zou W, Li S (2016) Microbial synthesis of poly-γ-glutamic acid: current progress, challenges, and future perspectives. Biotechnol Biofuels 9:134–145
Bajaj I, Singhal R (2011) Poly(glutamic acid)—an emerging biopolymer of commercial interest. Bioresour Technol 102:5551–5561
Inbaraj BS, Chiu YT, Chiu CP, Ho GH, Yang J, Chen BH (2006) Effect of pH on binding of mutagenic heterocyclic amines by the natural biopolymer poly(γ-glutamic acid). J Agric Food Chem 54:6452–6459
Inbaraj BS, Chien JT, Ho GH, Yang J, Chen BH (2006) Equilibrium and kinetic studies on sorption of basic dyes by a natural biopolymer poly(γ-glutamic acid). Biochem Eng J 31:204–215
Inbaraj BS, Chiu CP, Ho GH, Yang J, Chen BH (2006) Removal of cationic dyes from aqueous solution using an anionic poly(γ-glutamic acid)-based adsorbent. J Hazard Mater B137:226–234
Inbaraj BS, Chiu CP, Ho GH, Yang J, Chen BH (2008) Effects of temperature and pH on adsorption of basic brown 1 by the bacterial biopolymer poly(γ-glutamic acid). Bioresour Technol 99:1026–1035
Jamiu ZA, Saleh TA, Ali SA (2017) Biogenic glutamic acid-based resin: its synthesis and application in the removal of cobalt(II). J Hazard Mater 327:44–54
Hu P, Zhang Z, Shen F, Yu X, Li M, Nib H, Li L (2017) Poly-γ-glutamic acid coupled Pseudomonas putida cells surface-displaying metallothioneins: composited copper(II) biosorption and inducible flocculation in aqueous solution. RSC Adv 7:18578–18587
Bodnár M, Kjøniksen AL, Molnár RM, Hartmann JF, Daroczi L, Nystrom B, Borbely J (2008) Nanoparticles formed by complexation of poly-γ-glutamic acid with lead ions. J Hazard Mater 153:1185–1192
Zhang S, Zhang C, Liu M, Huang R, Su R, Qi W, He Z (2018) Poly(γ-glutamic acid) promotes enhanced dechlorination of p-chlorophenaol by Fe–Pd nanoparticles. Nanoscale Res Lett 13:219
Karmaker S, Saha TK (2008) Chelation of vanadium(IV) by a natural and edible biopolymer poly(γ-glutamic acid) in aqueous solution: Structure and binding constant of complex. Macromol Biosci 8:171–176
Karmaker S, Saha TK, Yoshikawa Y, Yasui H, Sakurai H (2006) A novel drug delivery system for type 1 diabetes: insulin-mimetic vanadyl-poly(γ-glutamic acid) complex. J Inorg Biochem 100:1535–1546
Karmaker S, Saha TK, Yoshikawa Y, Sakurai H (2007) Amelioration of hyperglycemia and metabolic syndromes in type 2 diabetic KKAy mice by poly(γ-glutamic acid)oxovanadium(IV) complex. ChemMedChem 2:1607–1612
Karmaker S, Saha TK, Yoshikawa Y, Sakurai H (2009) A zinc(II)/poly(γ-glutamic acid) complex as an oral therapeutic for the treatment of type-2 diabetic KKAy mice. Macromol Biosci 9:279–286
Sirelkhatim A, Mahmud S, Seeni A, Kaus NHM, Ann LC, Bakhori SKM, Hasan H, Mohamad D (2015) Review on zinc oxide nanoparticles: antibacterial activity and toxicity mechanism. Nanomicro Lett 7:219–242
Owoseni AA, Ayansina AD, Amjo OD (2015) In vitro assessment of the antimicrobial activities of leaf and stem extracts of Alchornea cordifolia. J Appl Sci Environ Manag 19:303–308
Bauer AW, Kirby WM, Sherris JC, Turck M (1966) Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol 45:493–496
Mondal R, Polash SA, Saha T, Islam Z, Sikder MM, Alam N, Hossain MS, Sarker SR (2017) Investigation of the phytoconstituents and bioactivity of various parts of wild type and cultivated Phyllanthus emblica L. Adv Biosci Biotechnol 8:211–227
Polash SA, Saha T, Hossain MS, Sarker SR (2017) Investigation of the phytochemicals, antioxidant, and antimicrobial activity of the Andrographis paniculata leaf and stem extracts. Adv Biosci Biotechnol 8:149–162
Zupancic M, Turel I, Bukovec P, White AJP, Williams DJ (2001) Synthesis and characterization of two novel zinc(II) complexes with ciprofloxacin. Crystal structure of [C17H19N3O3F]2·[ZnCl4]·2H2O. Croat Chem Acta 74:61–74
Deacon GB, Phillips RJ (1980) Relationships between the carbon–oxygen stretching frequencies of carboxylate complexes and the type of carboxylate coordination. Coord Chem Rev 33(1980):227–250
Siao FY, Lu JF, Wang JS, Inbaraj BS, Chen BH (2009) In vitro binding of heavy metals by an edible biopolymer poly(γ-glutamic acid). J Agric Food Chem 57:777–784
Inbaraj BS, Wang JS, Lu JF, Siao FY, Chen BH (2009) Adsorption of toxic mercury(II) by an extracellular biopolymer poly(γ-glutamic acid). Bioresour Technol 100:200–207
Goodinga EA, Sharma S, Petty SA, Fouts EA, Palmer CJ, Nolan BE, Volk M (2013) pH-dependent helix folding dynamics of poly-glutamic acid. Chem Phys 422:115–123
Ho G-H, Ho T-I, Hsieh K-H, Su Y-C, Lin P-Y, Yang J, Yang K-H, Yang S-C (2006) γ-Polyglutamic acid produced by Bacillus subtilis (natto): structural characteristics, chemical properties and biological functionalities. J Chin Chem Soc 53:1363–1384
Shim J-Y, Gupta VK (2007) Reversible aggregation of gold nanoparticles induced by pH dependent conformational transitions of a self-assembled polypeptide. J Colloid Interface Sci 316:977–983
Kurotu T (1992) Polarographic behavior of Cd2+ and Zn2+–PGA complexes. Polarographic and circular dichroism spectroscopic studies on the Cd2+ and/or Zn2+–poly(α-l-glutamic acid) complex in aqueous solution. Inorg Chim Acta 191:141–147
Mu RM, Zhang Z, Li XC, Liu D, Zhao YL (2018) Preparation of new biosorbents based on poly-γ-glutamic acid and its adsorption of heavy metal ions. IOP Conf Ser Earth Environ Sci 191:012061
Lagergren SY (1898) Zur theorie der sogenannten adsorption geloster stoffe. K Sven Vetenskapsakad Handl 24:1–39
McKay G, Ho YS (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465
Elovich SY, Larinov OG (1962) Theory of adsorption from solutions of nonelectrolytes on solid (I) equation adsorption from solutions and the analysis of its simplest Form, (II) verification of the equation of adsorption isotherm from solutions. Izv Akad Nauk SSSR Otd Khim Nauk 2:209–216
Karmaker S, Sen T, Saha TK (2015) Adsorption of reactive yellow 145 onto chitosan in aqueous solution: kinetic modeling and thermodynamic analysis. Polym Bull 72:1879–1897
Weber WJ, Morris JC (1963) Kinetics of adsorption on carbon from solution. J Sanit Eng Div Proc Am Soc Civ Eng 89:31–60
Freundlich H (1906) Adsorption solution. Z Phys Chem 57:384–470
Temkin MI, Pyzhev V (1940) Kinetics of ammonia synthesis on promoted iron catalyst. Acta Physicochim URSS 12:327–356
Langmuir I (1918) Adsorption of gases on plain surfaces of glass mica platinum. J Am Chem Soc 40:1361–1403
Mousa NE, Simonescu CM, Pătescu R-E, Onose C, Tardei C, Culiţă DC, Oprea O, Patroi D, Lavric V (2016) Pb2+ removal from aqueous synthetic solutions by calcium alginate and chitosan coated calcium alginate. React Funct Polym 109:137–150
Liu Y (2006) Some consideration on the Langmuir isotherm equation. Colloid Surf A Physicochem Eng Asp 274:34–36
Karmaker S, Sintaha F, Saha TK (2019) Kinetics, isotherm and thermodynamic studies of the adsorption of reactive red 239 dye from aqueous solution by chitosan 8B. Adv Biol Chem 9:1–22
Faiz U, Butt T, Satti L, Hussain W, Hanif F (2011) Efficacy of zinc as an antibacterial agent against enteric bacterial pathogens. J Ayub Med Coll Abbottabad 23:18–21
Ajayeoba TA, Dula S, Ijabadeniyi OA (2019) Properties of poly-γ-glutamic acid producing-Bacillus species isolated from Ogi liquor and lemon-Ogi liquor. Front Microbiol 10:771. https://doi.org/10.3389/fmicb.2019.00771
Acknowledgements
We gratefully acknowledge the support given to Prof. Dr. Tapan Kumar Saha by the University Grants Commission of Bangladesh to carry out this work (FY 2017-2018). We are thankful to Mr. Nikhil Chandra Bhoumik (Wazed Miah Science Research Center, Jahangirnagar University) for assisting in the use the atomic absorption spectroscopy (AAS) at the center.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Akter, B., Khan, A.I., Karmaker, S. et al. Chelation of zinc(II) with poly(γ-glutamic acid) in aqueous solution: kinetics, binding constant, and its antimicrobial activity. Polym. Bull. 78, 1353–1377 (2021). https://doi.org/10.1007/s00289-020-03165-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-020-03165-9