Abstract
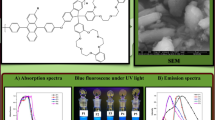
Rapid synthesis of new fluorene-based conjugated polymers P1–P8 is reported by polymerization of polyphenylene-substituted dendronized monomer 2,7-dibromo-9,9-di(4-(2,3,4,5-tetraphenylphenyl)-benzyl) fluorene (M1) and 2,7-dibromo-9,9-di(4-pentaphenylphenyl)-benzyl) fluorene (M2) with different 9,9-disubstituted 2,7-dibromo fluorene monomers (M3–M6) under microwave irradiation. The structure of these synthesized polymers P1–P8 was established by FTIR, 1H NMR, 13C NMR, and gel permeation chromatography techniques. The photophysical studies of these polymers P1–P8 shows good results desirable for light-emitting material. These polymers exhibited UV–Vis absorption peak with the maxima in 344–386 nm in THF solution. Similarly, the fluorescence spectra of these polymers showed PL maxima in 414–418 nm with shoulder peak in 437–440 nm. From this study, the stoke shifts was observed in 30–73 nm, and quantum efficiency was found in 0.41–0.57. Polymers had thermal stability up to 200 °C; however, for dihexyl-substituted dendronized polymer, P1 and P5 showed thermal decomposition at 490 and 430 °C, respectively. In addition to this, polymers P1–P8 were also analyzed by electrochemical study in which the onset of the irreversible oxidation wave of dendronized polymers P1–P8 is recorded in the range of 0.88–0.99 V. The results of these various studies showed that the synthesized polymers P1–P8 can be promising materials for blue-light-emitting diodes because of their high photoluminescence (PL), quantum efficiencies, and thermal stability.







Similar content being viewed by others
References
Hains AW, Liang Z, Woodhouse MA, Gregg BA (2010) Molecular semiconductors in organic photovoltaic cells. Chem Rev 110:6689–6735. https://doi.org/10.1021/cr9002984
Jiang L, Dong H, Hu W (2010) Organic single crystal field-effect transistors: advances and perspectives. J Mater Chem 20:4994–5007. https://doi.org/10.1039/B925875B
Grimsdale AC, Chan KL, Martin RE (2009) Synthesis of light-emitting conjugated polymers for applications in electroluminescent devices. Chem Rev 109:897–1091. https://doi.org/10.1021/cr000013v
Cho HJ, Jung BJ, Cho NS (2003) Synthesis and characterization of thermally stable blue light-emitting polyfluorenes containing siloxane bridges. Macromolecules 2003; 36: 6704–6710. Macromolecules 36:6704–6710. https://doi.org/10.1021/ma034622r
Beaujuge PM, Reynolds JR (2010) Color control in pi-conjugated organic polymers for use in electrochromic devices. Chem Rev 110:268–320. https://doi.org/10.1021/cr900129a
Klauk H (2010) Organic thin-film transistors. Chem Soc Rev 39:2643–2666. https://doi.org/10.1039/B909902F
Burroughes JH, Bradley DDC, Brown AR, Marks RN, Mackay K, Friend RH, Burns PL, Holmes AB (1990) Light-emitting diodes based on conjugated polymers. Nature 347:539–541. https://doi.org/10.1038/347539a0
Tomalia DA, Frechet JMJ (2002) Discovery of dendrimers and dendritic polymers: a brief historical perspective. J Polym Sci, Part A: Polym Chem 40:2719–2728. https://doi.org/10.1002/pola.10301
Hawker CJ, Frechet JM (1992) The synthesis and polymerization of a hyperbranched polyether macromonomer. Polymer 33:1507–1511. https://doi.org/10.1016/0032-3861(92)90128-J
Percec V, Heck J, Tomazos D, Falkenberg F, Blackwell H, Ungar G (1993) Self-assembly of taper-shaped monoesters of oligo(ethylene oxide) with 3,4,5-tris(p-dodecyloxybenzyloxy)benzoic acid and of their polymethacrylates into tubular supramolecular architectures displaying a columnar mesophase. J Chem Soc 1:2799–2811. https://doi.org/10.1039/P19930002799
Peng Q, Huang Y, Cao Y (2004) Synthesis and characterization of new red-emitting polyfluorene derivatives containing electron-deficient 2-pyran-4-ylidene − malononitrile moieties. Macromolecules 37:260–266. https://doi.org/10.1021/ma0355397
Ranger M, Rondeau D, Leclerc M (1997) New well-defined poly(2,7-fluorene) derivatives: photoluminescence and base doping. Macromolecules 30:7686–7691. https://doi.org/10.1021/ma970920a
Grisorio R, Suranna GP, Mastrorilli P (2007) Insight into the role of oxidation in the thermally induced green band in fluorene-based systems. Adv Funct Mater 17:538–548. https://doi.org/10.1002/adfm.200600083
Sandee AJ, Williams CK, Evans NR, Davies JE, Boothby CE, Kohler A, Friend RH, Holmes AB (2004) Solution-processible conjugated electrophosphorescent polymers. J Am Chem Soc 126:7041–7048. https://doi.org/10.1021/ja039445o
Pinner DJ, Friend RH, Tessler N (1999) Transient electroluminescence of polymer light emitting diodes using electrical pulses. J Appl Phys 86:5116–5130. https://doi.org/10.1063/1.371488
Charas A, Morgado J, Martinho JMG, Alcacer LS, Lim F, Friend RH, Cacialli F (2003) Synthesis and luminescence properties of three novel polyfluorene copolymers. Polymer 44:1843–1850. https://doi.org/10.1016/S0032-3861(03)00028-4
Bezgin B, Cihaner A, Onal AM (2008) Electrochemical polymerization of 9-fluorenecarboxylic acid and its electrochromic device application. Thin Solid Films 516:7329–7334. https://doi.org/10.1016/j.tsf.2008.02.003
Tsuie B, Reddinger JL, Sotzing GA, Soloducho J, Katritzky AR, Reynolds JR (1999) Electroactive and luminescent polymers: new fluorene-heterocycle-based hybrids. J Mater Chem 9:2189–2200. https://doi.org/10.1039/A903374B
Çarbas BB, Kivrak A, Onal AM (2012) A new processable electrochromic polymer based on an electron deficient fluorene derivative with a high coloration efficiency. Electrochim Acta 58:223–234. https://doi.org/10.1016/j.electacta.2011.09.037
Larmat F, Reynolds JR, Reinhardt BA, Brott LL, Clarson SI (1997) Comparative reactivity of thiophene and 3,4-(ethylenedioxy)thiophene as terminal electropolymerizable units in bis-heterocycle arylenes. J Polym Sci Part A Polym Chem 35:3627–3636. https://doi.org/10.1002/(sici)1099-0518(199712)35:17<3627::aid-pola2>3.0.co;2-p
Nie G, Yang H, Chen J, Bai Z (2012) A novel high-quality electrochromic material from 3,4-ethylenedioxythiophene bis-substituted fluorine. Org Electron 13:2167–2176. https://doi.org/10.1016/j.orgel.2012.05.055
Ibrahimova V, Kocak ME, Onal AM, Tuncel D (2013) Optical and electronic properties of fluorene-based copolymers and their sensory applications. J Polym Sci, Part A: Polym Chem 51:815–823. https://doi.org/10.1002/pola.26454
Keivanidis PE, Howard IA, Friend RH (2008) Intermolecular interactions of perylene diimides in photovoltaic blends of fluorene copolymers: disorder effects on photophysical properties, film morphology and device efficiency. Adv Funct Mater 18:3189–3202. https://doi.org/10.1002/adfm.200800356
Klaerner G, Davey MH, Chen WD, Scott JC, Miller RD (1998) Colorfast blue-light-emitting random copolymers derived from di-n-hexylfluorene and anthracene. Adv Mater 10:993–997. https://doi.org/10.1002/(sici)1521-4095(199809)10:13<993::aid-adma993>3.0.co;2-2
Klaerner G, Lee JI, Davey MH, Miller RD (1999) Exciton migration and trapping in copolymers based on dialkylfluorenes. Adv Mater 11:115–119. https://doi.org/10.1002/(sici)1521-4095(199902)11:2<115::aid-adma115>3.0.co;2-n
Klarner G, Lee JI, Lee VY, Chan E, Chen JP, Nelson A, Markiewicz D, Siemens R, Scott JC, Miller RD (1999) Cross-linkable polymers based on dialkylfluorenes. Chem Mater 11:1800–1805. https://doi.org/10.1021/cm990027l
Liu J, Tu G, Zhou Q, Cheng Y, Geng Y, Wang L, Ma D, Jing X, Wang FJ (2006) Highly efficient green light emitting polyfluorene incorporated with 4-diphenylamino-1,8-naphthalimide as green dopant. J Mater Chem 16:1431–1438. https://doi.org/10.1039/B514359D
Evans NR, Devi LS, Mak CSK, Watkins SE, Pascu SI, Kohler A, Friend RH, Williams CK, Holmes AB (2006) Triplet energy back transfer in conjugated polymers with pendant phosphorescent iridium complexes. J Am Chem Soc 128:6647–6656. https://doi.org/10.1021/ja0584267
Setayesh S, Grimsdale AC, Weil T, Enkelmann V, Mullen K, Meghdadi F, List EJ, Leising GJ (2001) Polyfluorenes with polyphenylene dendron side chains: toward non-aggregating, light-emitting polymers. J Am Chem Soc 123:946–953. https://doi.org/10.1021/ja0031220
Leclerc M (2001) Polyfluorenes: twenty years of progress. J Polym Sci, Part A: Polym Chem 39:2867–2873. https://doi.org/10.1002/pola.1266
Neher D, (2001) Polyfluorene homopolymers: conjugated liquid-crystalline polymers for bright blue emission and polarized electroluminescence. Macromol Rapid Commun 22:1365–1385. https://doi.org/10.1002/1521-3927(20011101)22:17<1365::aid-marc1365>3.0.co;2-b
Woo EP, Shiang WR, Inbasekaran M, Roof GR, Bernius MT, Weishi W (2005) Fluorene-containing polymers and compounds useful in the preparation thereof, US Patent, US6900285
Williams JAG (2007) Organic light-emitting devices: synthesis, properties and applications. Platin Metals Rev 51:85–86. https://doi.org/10.1595/147106707x192537
Newkome GR, Moorefield CN, Vögtle F (2001) Dendrimers and dendrons: concepts, syntheses, application. Wiley, New York
Kenneth RC (2002) Nickel(0)-mediated coupling polymerizations via microwave-assisted chemistry. Macromolecules 35:6757–6759. https://doi.org/10.1021/ma025515k
Kadu RK, Patil VR (2015) new strategy for synthesis of polyphenylene substituted dendronized monomers containing fluorene unit and the study of their properties. Polycycl Aromat Compd. https://doi.org/10.1080/10406638.2015.1129974
Saikia G, Iyer PK (2010) Facile C–H alkylation in water: enabling defect-free materials for optoelectronic devices. J Org Chem 75:2714–2717. https://doi.org/10.1021/jo100028d
Hoz A, Ortiz AD, Moreno A (2005) Microwaves in organic synthesis. Thermal and non-thermal microwave effects. Chem Soc Rev 34:164–178. https://doi.org/10.1039/B411438H
Kappe CO (2004) Controlled microwave heating in modern organic synthesis. Angew Chem Int Ed 43:6250–6284. https://doi.org/10.1002/anie.200400655
Lidstrom P, Tierney J, Wathey B, Westman J (2001) Microwave assisted organic synthesis—a review. Tetrahedron 57:9225–9283. https://doi.org/10.1016/S0040-4020(01)00906-1
Nuchter M, Ondruschka B, Bonrath W, Gum A (2004) Microwave assisted synthesis—a critical technology overview. Green Chem 6:128–141. https://doi.org/10.1039/B310502D
Shinpei MY, Susumu T, Junichi S, Kenji M, Shunzo S, Kenji T (2009) Microwave-assisted preparation of poly(fluorene)s by Ni-catalyzed polymerization. Polym J 4:327–331. https://doi.org/10.1295/polymj.PJ2008330
Matthias B, Luisa D, Bozano J, Campbell S, Kenneth RC (2005) Design and synthesis of new polymeric materials for organic nonvolatile electrical bistable storage devices: poly(biphenylmethylene)s. Macromolecules 38:4147–4156. https://doi.org/10.1021/ma049572k
Yoan CS, Joseph JP, Christine M, Kenneth RC, Bryan CE (2009) Synthesis of polyfluorenes with pendant silylcarboranes. Macromolecules 42:512–516. https://doi.org/10.1021/ma8018816
Acknowledgements
The author (RKK) would like to acknowledge the University of Mumbai, for providing the financial support under the UGC scheme.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kadu, R.K., Thakur, P.B. & Patil, V.R. Photophysical properties of new fluorene-based conjugated polymers containing polyphenylene-substituted dendronized core. Polym. Bull. 76, 595–613 (2019). https://doi.org/10.1007/s00289-018-2401-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-018-2401-3