Abstract
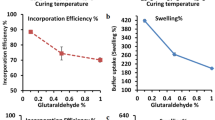
To improve the durability and bioavailability of alginate, a one-step extrusion method was successfully applied to prepare alginate/gelatin core–shell beads. Gelatin cross-linked with glutaraldehyde was used as the core, while sodium alginate was used as the shell. To evaluate the effect of the sodium alginate shell on the in vitro drug release properties of the beads for biomedical applications, two drug models were used: water-soluble metformin hydrochloride and water-insoluble indomethacin. The structure and properties of different core–shell beads were characterized by Fourier transform infrared spectroscopy, scanning electron microscopy and swelling tests. The results showed that the beads consist of obvious inner core and outer skin layer which coats the surface stably. In addition, the core–shell structure improved the thermal stability of the beads and the entrapment efficiency reached 90% with a sodium alginate shell. As demonstrated, there is a gradual decrease in the swelling degree as the GTA or alginate concentration increases. For indomethacin, the cumulative release was 8.7% after 360 min in HCl buffer. The anomalous transport mechanism was the predominant factor affecting the release behavior of the metformin hydrochloride-loaded beads and indomethacin loaded beads were controlled by case-II transport. This work suggests that the core–shell structure could improve the swelling properties and drug release behavior of the beads.









Similar content being viewed by others
References
Poojari R, Srivastava R (2013) Composite alginate microspheres as the next-generation egg-box carriers for biomacromolecules delivery. Expert Opin Drug Deliv 10:1061–1076
Chen Y, Chen H et al (2010) Core shell structured hollow mesoporous nanocapsules: a potential platform for simultaneous cell imaging and anticancer drug delivery. ACS Nano 4:6001–6013
Sun J, Tan H (2013) Alginate-based biomaterials for regenerative medicine applications. Materials 6:1285–1309
Sagiri SS, Singh VK et al (2015) Core–shell-type organogel–alginate hybrid microparticles: a controlled delivery vehicle. Chem Eng J 264:134–145
Matricardi P, Di Meo C et al (2013) Interpenetrating polymer networks polysaccharide hydrogels for drug delivery and tissue engineering. Adv Drug Deliv Rev 65:1172–1187
Islan GA, Castro GR (2014) Tailoring of alginate–gelatin microspheres properties for oral Ciprofloxacin-controlled release against Pseudomonas aeruginosa. Drug Deliv 21:615–626
Lee KY, Mooney DJ (2012) Alginate: properties and biomedical applications. Prog Polym Sci 37:106–126
Yao R, Zhang R et al (2012) Alginate and alginate/gelatin microspheres for human adipose-derived stem cell encapsulation and differentiation. Biofabrication 4:025007
Sakai S, Yamaguchi S et al (2008) Oxidized alginate-cross-linked alginate/gelatin hydrogel fibers for fabricating tubular constructs with layered smooth muscle cells and endothelial cells in collagen gels. Biomacromol 9:2036–2041
Thu HE, Ng SF (2013) Gelatine enhances drug dispersion in alginate bilayer film via the formation of crystalline microaggregates. Int J Pharm 454:99–106
Sarbon NM, Badii F et al (2013) Preparation and characterisation of chicken skin gelatin as an alternative to mammalian gelatin. Food Hydrocoll 30:143–151
Michon C, Cuvelier G et al (1993) Concentration dependence of the critical viscoelastic properties of gelatin at the gel point. Rheol Acta 32:94–103
Hiwale P, Lampis S et al (2011) In vitro release of lysozyme from gelatin microspheres: effect of cross-linking agents and thermoreversible gel as suspending medium. Biomacromol 12:3186–3193
Mariod AA, Adam HF (2013) Review: gelatin, source, extraction and industrial applications. Acta Sci Pol Technol Aliment 12:135–147
Paques JP, van der Linden E et al (2014) Preparation methods of alginate nanoparticles. Adv Colloid Interface Sci 209:163–171
Wu C, Fan W et al (2011) In situ preparation and protein delivery of silicate–alginate composite microspheres with core–shell structure. J R Soc Interface 8:1804–1814
Lim MPA, Lee WL et al (2013) One-step fabrication of core–shell structured alginate-PLGA/PLLA microparticles as a novel drug delivery system for water soluble drugs. Biomater Sci 1:486–493
Perez RA, Kim HW (2015) Core–shell designed scaffolds for drug delivery and tissue engineering. Acta Biomater 21:2–19
Poudel BK, Pradhan R et al (2014) Preparation and characterization of alginate gel core-lipid nanocapsules for co-delivery of hydrophilic and hydrophobic anti-cancer drugs. J Pharm Investig 44:485–491
Haidar ZS, Azari F et al (2009) Modulated release of OP-1 and enhanced preosteoblast differentiation using a core–shell nanoparticulate system. J Biomed Mater Res A 91:919–928
Del Gaudio P, Auriemma G et al (2014) Novel co-axial prilling technique for the development of core–shell particles as delayed drug delivery systems. Eur J Pharm Biopharm 87:541–547
Yang CH, Wang CY et al (2014) Core–shell structure microcapsules with dual pH-responsive drug release function. Electrophoresis 35:2673–2680
Huang KS, Yang CH et al (2014) Synthesis of uniform core–shell gelatin-alginate microparticles as intestine-released oral delivery drug carrier. Electrophoresis 35:330–336
Zheng X, Smit M et al (2005) Fragmentation behavior of silica-supported metallocene/MAO catalyst in the early stages of olefin polymerization. Macromolecules 38:4673–4678
Mi FL, Sung HW et al (2002) Drug release from chitosan–alginate complex beads reinforced by a naturally occurring cross-linking agent. Carbohydr Polym 48:61–72
Saarai A, Kasparkova V et al (2013) On the development and characterisation of crosslinked sodium alginate/gelatine hydrogels. J Mech Behav Biomed 18:152–166
Kumbar SG, Soppimath KS et al (2003) Synthesis and characterization of polyacrylamide-grafted chitosan hydrogel microspheres for the controlled release of indomethacin. J Appl Polym Sci 87:1525–1536
Işiklan N (2006) Controlled release of insecticide carbaryl from sodium alginate, sodium alginate/gelatin, and sodium alginate/sodium carboxymethyl cellulose blend beads crosslinked with glutaraldehyde. J Appl Polym Sci 99:1310–1319
Kumar P, Singh I (2010) Formulation and characterization of tramadol-loaded IPN microgels of alginate and gelatin: optimization using response surface methodology. Acta Pharm 60:295–310
Dong Z, Wang Q et al (2006) Alginate/gelatin blend films and their properties for drug controlled release. J Membr Sci 280:37–44
Devi N, Kakati DK (2013) Smart porous microparticles based on gelatin/sodium alginate polyelectrolyte complex. J Food Eng 117:193–204
Saito T, Kuramaeet R et al (2013) An ultrastrong nanofibrillar biomaterial: the strength of single cellulose nanofibrils revealed via sonication-induced fragmentation. Biomacromol 14:248–253
Li L, Li J et al (2015) Effect of formulation variables on in vitro release of a water-soluble drug from chitosan–sodium alginate matrix tablets. Asian J Pharm Sci 10:314–321
Peppas N (1985) Analysis of Fickian and non-Fickian drug release from polymers. Pharm Acta Helv 60:110–111
Krstić J, Spasojević J et al (2014) In vitro silver ion release kinetics from nanosilver/poly(vinyl alcohol) hydrogels synthesized by gamma irradiation. J Appl Polym Sci 131:40321
Lin N, Huang J et al (2011) Effect of polysaccharide nanocrystals on structure, properties, and drug release kinetics of alginate-based microspheres. Colloids Surf B 85:270–279
Acknowledgements
The financial support from the National Natural Science Foundation Commission of China (Nos. 51373158, 51673177) and the Sci-Tech. Innovation Talent Foundation of Henan Province (No. 144200510018) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guo, T., Zhang, N., Huang, J. et al. A facile fabrication of core–shell sodium alginate/gelatin beads for drug delivery systems. Polym. Bull. 76, 87–102 (2019). https://doi.org/10.1007/s00289-018-2377-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-018-2377-z