Abstract
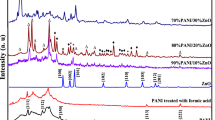
A new ternary nanocomposite of polyaniline (PANI), prussian blue (PB) and zinc oxide (ZnO), i.e., PANI–PB–ZnO was successfully synthesized in one pot process through chemical oxidative polymerization of aniline using ammonium persulfate as oxidizing agent. The morphological studies showed the formation of PB nanocubes and agglomerated quasi-spherical ZnO nanoparticles over PANI matrix, and the elemental composition of PANI–PB–ZnO was analyzed with energy-dispersive X-ray spectroscopy. The XRD measurements reveal the semicrystalline structure of the PANI–PB–ZnO nanocomposite after the polymerization reaction. The various functional groups present in PANI–PB–ZnO were identified using FTIR spectroscopy which confirms the presence of ZnO, PB and PANI in the synthesized ternary nanocomposite. From thermogravimetric analysis, the thermal degradation mechanism of PANI–PB–ZnO nanocomposite was explored and the activation energy (EA) was calculated from Coats–Redfern plot and was found to be 14.33 kJ/mol. 38 wt% of PANI–PB–ZnO nanocomposite was obtained as a residue at 800 °C indicating the high thermal stability. The bulk ionic conductivity value of the synthesized nanocomposite was found to be 8.509 × 10−5 Ω−1 cm−1 at 30 °C and 1.22 × 10−4 Ω−1 cm−1 at 100 °C. The ionic conductivity was found to increase with temperature for the synthesized material which showed an increase in the number of effective charge carriers. The characteristic absorption bands were detected using UV–Vis spectroscopy that confirms the formation of PANI–PB–ZnO nanocomposite. X-ray photoelectron spectroscopy measurements confirmed the valence states of constituent elements in ZnO nanoparticles and ternary PANI–PB–ZnO nanocomposite. I–V studies revealed that hybrid PANI–PB–ZnO nanocomposite has higher conductivity (2.4 × 10−4 S cm−1) than ZnO nanoparticles (3.5 × 10−10 S cm−1).











Similar content being viewed by others
References
Reddy KR, Karthik KV, Prasad SB, Soni SK, Jeong HM, Raghu AV (2016) Enhanced photocatalytic activity of nanostructured titanium dioxide/polyaniline hybrid photocatalysts. Polyhedron 120:169–174
Reddy KR, Lee KP, Lee Y, Gopalan AI (2008) Facile synthesis of conducting polymer–metal hybrid nanocomposite by in situ chemical oxidative polymerization with negatively charged metal nanoparticles. Mater Lett 62:1815–1818
Hu C, Li Y, Zhang N, Ding Y (2017) Synthesis and characterization of a poly(o-anisidine)–SiC composite and its application for corrosion protection of steel. RSC Adv 7:11732–11742
Reddy KR, Lee KP, Gopalan AI (2007) Self-assembly directed synthesis of poly(ortho-toluidine)-metal (gold and palladium) composite nanospheres. J Nanosci Nanotechnol 7:3117–3125
Ameen S, Akhtar MS, Ansari SG, Yang OB, Shin HS (2009) Electrophoretically deposited polyaniline/ZnO nanoparticles for p–n heterostructure diodes. Superlattices Microstruct 46:872–880
Sathiyanarayanan S, Azim SS, Venkatachari G (2007) Preparation of polyaniline–TiO2 composite and its comparative corrosion protection performance with polyaniline. Synth Met 157:205–213
Stejskal J, Sapurina I, Trchová M (2010) Polyaniline nanostructures and the role of aniline oligomers in their formation. Prog Polym Sci 35:1420–1481
Zhang YP, Lee SH, Reddy KR, Gopalan AI, Lee KP (2007) Synthesis and characterization of core-shell SiO2 nanoparticles/poly(3-aminophenylboronic acid) composites. J Appl Polym Sci 104:2743–2750
Reddy KR, Lee KP, Gopalan AI (2007) Novel electrically conductive and ferromagnetic composites of poly(aniline-co-aminonaphthalenesulfonic acid) with iron oxide nanoparticles: synthesis and characterization. J Appl Polym Sci 106:1181–1191
Reddy KR, Lee KP, Gopalan AI, Showkat AM (2006) Facile synthesis of hollow spheres of sulfonated polyanilines. Polym J 38:349–354
Hassan M, Reddy KR, Haque E, Faisal SN, Ghasemi S, Minett AI, Gomes VG (2014) Hierarchical assembly of graphene/polyaniline nanostructures to synthesize free-standing supercapacitor electrode. Compos Sci Technol 98:1–8
Rodrigues R, Ferreira Q, Mendonça AL, Morgado J (2014) Template role of polyhexylthiophene nanowires on efficient bilayer photovoltaic cells. Synth Met 190:72–78
Chiang JC, MacDiarmid AG (1986) ‘Polyaniline’: protonic acid doping of the emeraldine form to the metallic regime. Synth Met 13:193–205
Reddy KR, Park W, Sin BC, Noh J, Lee Y (2009) Synthesis of electrically conductive and superparamagnetic monodispersed iron oxide-conjugated polymer composite nanoparticles by in situ chemical oxidative polymerization. J Colloid Interface Sci 335:34–39
Le Goff A, Holzinger M, Cosnier S (2011) Enzymatic biosensors based on SWCNT-conducting polymer electrodes. Analyst 136:1279–1287
Ohyama M, Kouzuka H, Yoko T (1997) Sol-gel preparation of ZnO films with extremely preferred orientation along (002) plane from zinc acetate solution. Thin Solid Films 306:78–85
Jin ZC, Hamberg I, Granqvist CG, Sernelius BE, Berggren KF (1988) Reactively sputtered ZnO:Al films for energy-efficient windows. Thin Solid Films 164:381–386
Spanhel L, Anderson MA (1991) Semiconductor clusters in the sol–gel process: quantized aggregation, gelation, and crystal growth in concentrated zinc oxide colloids. J Am Chem Soc 113:2826–2833
Chu SY, Yan TM, Chen SL (2000) Characteristics of sol–gel synthesis of ZnO-based powders. J Mater Sci Lett 19:349–352
Tokumoto MS, Briois V, Santilli CV, Pulcinelli SH (2003) Preparation of ZnO nanoparticles: structural study of the molecular precursor. J Sol-Gel Sci Technol 26:547–551
Kim JH, Choi WC, Kim HY, Kang Y, Park YK (2005) Preparation of mono-dispersed mixed metal oxide micro hollow spheres by homogeneous precipitation in a micro precipitator. Powder Technol 153:166–175
Damonte LC, Zélis LM, Soucase BM, Fenollosa MH (2004) Nanoparticles of ZnO obtained by mechanical milling. Powder Technol 148:15–19
Kahn ML, Monge M, Collière V, Senocq F, Maisonnat A, Chaudret B (2005) Size-and shape-control of crystalline zinc oxide nanoparticles: a new organometallic synthetic method. Adv Funct Mater 15:458–468
Komarneni S, Bruno M, Mariani E (2000) Synthesis of ZnO with and without microwaves. Mater Res Bull 35:1843–1847
Zhao X, Zheng B, Li C, Gu H (1998) Acetate-derived ZnO ultrafine particles synthesized by spray pyrolysis. Powder Technol 100:20–23
Tani T, Mädler L, Pratsinis SE (2002) Homogeneous ZnO nanoparticles by flame spray pyrolysis. J Nanopart Res 4:337–343
Dai ZR, Pan ZW, Wang ZL (2003) Novel nanostructures of functional oxides synthesized by thermal evaporation. Adv Funct Mater 13:9–24
Ao W, Li J, Yang H, Zeng X, Ma X (2006) Mechanochemical synthesis of zinc oxide nanocrystalline. Powder Technol 168:148–151
Kovtyukhova NI, Gorchinskiy AD, Waraksa C (2000) Self-assembly of nanostructured composite ZnO/polyaniline films. Mater Sci Eng B 69:424–430
He Y (2005) A novel emulsion route to sub-micrometer polyaniline/nano-ZnO composite fibers. Appl Surf Sci 249:1–6
Siddheswaran R, Sankar R, Ramesh Babu M, Rathnakumari M, Jayavel R, Murugakoothan P, Sureshkumar P (2006) Preparation and characterization of ZnO nanofibers by electrospinning. Cryst Res Technol 41:446–449
Mitra S, Patra P, Chandra S, Pramanik P, Goswami A (2012) Efficacy of highly water-dispersed fabricated nano ZnO against clinically isolated bacterial strains. Appl Nanosci 2:231–238
Lai Y, Meng MYuY, Wang X, Ding T (2011) Photoluminescence and photocatalysis of the flower-like nano-ZnO photocatalysts prepared by a facile hydrothermal method with or without ultrasonic assistance. Appl Catal B 105:335–345
Hussein MZB, Yun-Hin TY, Bin Tawang MM, Shahadan R (2002) Thermal degradation of (zinc–aluminium-layered double hydroxide-dioctyl sulphosuccinate) nanocomposite. Mater Chem Phys 74:265–271
Ghule K, Ghule AV, Chen BJ, Ling YC (2006) Preparation and characterization of ZnO nanoparticles coated paper and its antibacterial activity study. Green Chem 8:1034–1041
Wang Y, Wang RXuS, Liu Q, Wang J (2015) Polypyrrole/polyaniline composites with enhanced performance for capacitive deionization. Desalination Water Treat 54:3248–3256
Planche MF, Thieblemont JC, Mazars N, Bidan G (1994) Kinetic study of pyrrole polymerization with iron(III) chloride in water. J Appl Polym Sci 52:1867–1877
Wu C, Qiao X, Chen J, Wang H, Tan F, Li S (2006) A novel chemical route to prepare ZnO nanoparticles. Mater Lett 60:1828–1832
Wahab R, Kim YS, Lee K, Shin HS (2010) Fabrication and growth mechanism of hexagonal zinc oxide nanorods via solution process. J Mater Sci 45:2967–2973
Zhao QX, Klason P, Willander M (2007) Growth of ZnO nanostructures by vapor–liquid–solid method. Appl Phys A Mater Sci Process 88:27–30
Babaei-Dehkordi A, Moghaddam J, Mostafaei A (2013) An optimization study on the leaching of zinc cathode melting furnace slag in ammonium chloride by Taguchi design and synthesis of ZnO nanorods via precipitation methods. Mater Res Bull 48:4235–4247
Cullity BD, Stock SR (2001) Elements of X-ray diffraction. Prentice-Hall, Englewood Cliffs
Pant HC, Patra MK, Negi SC, Bhatia A, Vadera SR, Kumar N (2006) Studies on conductivity and dielectric properties of polyaniline–zinc sulphide composites. Bull Mater Sci 29:379–384
Vinayak GK, Chakradhar SB, Hussain J, Prasad MA (2015) Synthesis, characterization and DC conductivity studies on polyaniline/ZnO composites. Ferroelectrics 486:106–113
El-hadi AM, Al-Jabri FY, Altaf WJ (2017) Higher dielectric properties of semiconducting biopolymer composites of poly(3-hydroxy butyrate) (PHB) with polyaniline (PANI), carbon black, and plasticizer. Polym Bull. https://doi.org/10.1007/s00289-017-2118-8
Hao J, Zhao W, Zhang H, Wang D, Yang Q, Tang N, Wang X (2017) Controlled synthesis of PANI nanostructures using phenol and hydroquinone as morphology-control agent. Polym Bull. https://doi.org/10.1007/s00289-017-2159-z
Souquet JL, Levy M, Duclot M (1994) A single microscopic approach for ionic transport in glassy and polymer electrolytes. Solid State Ion 70:337–345
Wang JG, Yang Y, Huang ZH, Kang F (2012) Interfacial synthesis of mesoporous MnO2/polyaniline hollow spheres and their application in electrochemical capacitors. J Power Sources 204:236–243
Ma RENZHI, Bando Y, Zhang LIANQI, Sasaki T (2004) Layered MnO2 nanobelts: hydrothermal synthesis and electrochemical measurements. Adv Mater 16:918–922
Chang MY, Wu CS, Chen YF, Hsieh BZ, Huang WY, Ho KS, Hsieh TH, Han YK (2008) Polymer solar cells incorporating one-dimensional polyaniline nanotubes. Org Electron 9:1136–1139
Wang X, Li Y, Zhao Y, Liu J, Tang S, Feng W (2010) Synthesis of PANI nanostructures with various morphologies from fibers to micromats to disks doped with salicylic acid. Synth Met 160:2008–2014
Šeděnková I, Trchová M, Stejskal J (2008) Thermal degradation of polyaniline films prepared in solutions of strong and weak acids and in water–FTIR and Raman spectroscopic studies. Polym Degrad Stab 93:2147–2157
Zheng JH, Jiang Q, Lian JS (2011) Synthesis and optical properties of flower-like ZnO nanorods by thermal evaporation method. Appl Surf Sci 257:5083–5087
Moulder JF, Stickle WF, Sobol PE, Bomben KD (1992) Standard spectra for identification and interpretation of XPS data. Perkin Elmer Eden Prairie MN 89
Das J, Pradhan SK, Sahu DR, Mishra DK, Sarangi SN, Nayak BB, Verma S, Roul BK (2010) Micro-Raman and XPS studies of pure ZnO ceramics. Phys B Condens Matter 405:2492–2497
Qiu Z, Gong H, Yang X, Zhang Z, Han J, Cao B, Nakamura D, Okada T (2015) Phosphorus concentration dependent microstructure and optical property of ZnO nanowires grown by high-pressure pulsed laser deposition. J Phys Chem C 119:4371–4378
Moulder JF, Stickle WF, Sobol PE, Bomben KD (1992) Handbook of X-ray photoelectron spectroscopy. Perkin-Elmer Eden Prairie MN 52
Zhou H, Li Z (2005) Synthesis of nanowires, nanorods and nanoparticles of ZnO through modulating the ratio of water to methanol by using a mild and simple solution method. Mater Chem Phys 89:326–331
Zhang J, Gao J, Song Q, Guo Z, Chen A, Chen G, Zhou S (2016) N-substituted carboxyl polyaniline covalent grafting reduced graphene oxide nanocomposites and its application in supercapacitor. Electrochim Acta 199:70–79
Zou Y, Wang Q, Xiang C, She Z, Chu H, Qiu S, Xu F, Liu S, Tang C, Sun L (2016) One-pot synthesis of ternary polypyrrole–Prussian-blue–graphene-oxide hybrid composite as electrode material for high-performance supercapacitors. Electrochim Acta 188:126–134
Yu H, Jang K, Chung I, Ahn H (2016) Fabrication and electrochemical characterization of polyaniline/titanium oxide nanoweb composite electrode for supercapacitor application. J Nanosci Nanotechnol 16:2937–2943
Acknowledgements
The authors acknowledge the management of SSN College of Engineering, Kalavakkam, for the financial support provided in the current research work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Muthusamy, S., Charles, J. In situ synthesis and characterization of polyaniline/prussian blue/zinc oxide nanocomposite. Polym. Bull. 76, 119–137 (2019). https://doi.org/10.1007/s00289-018-2350-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-018-2350-x