Abstract
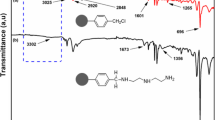
In this study, a new adsorbent was prepared to remove chromium (Cr(VI)) ions, which adversely affect human health even at low concentrations, from aqueous solutions and were characterized by FTIR, TGA, SEM, and BET analysis. The neutral pH point of adsorbent was determined. Later, the effects of initial ion concentration, pH, temperature, time, and amount of adsorbent on adsorption were investigated. Moreover, effect on Cr(VI) ion adsorption on both anions, such as nitrate and sulfate, and cations, such as lead, cadmium, and nickel, was also investigated. In addition, isotherm, kinetic and thermodynamic studies were also carried out. It was found that adsorption equilibrium time was 6 h, optimum pH was 2, the adsorption amount of chrome increased with increasing chrome concentration and increasing temperature. Cr(VI) ion adsorption data showed compliance with the best Langmuir isotherm and adsorption capacity of adsorbent was found to be 88.5, 103.1, and 107.5 mg/g at 298, 308, and 318 K, respectively. From kinetic data, adsorption of Cr(VI) onto developed adsorbent was found to comply with pseudo-second-order kinetic model. From the results of thermodynamic study, it was determined that the adsorption was endothermic and spontaneous. Cr(VI) ion adsorption of prepared adsorbent was not affected both in the presence of anions such as nitrate (NO3 −) and sulfate (SO4 2−) and in the presence of toxic metal ions such as Pb2+, Ni2+, and Cd2+ at pH 2, that is, the adsorbent was found to be selective for Cr(VI) ions. Furthermore, prepared adsorbent, applied to actual industrial wastewater, exhibited good performance for the removal of Cr(VI) ions.
















Similar content being viewed by others
References
Mohan D, Pittman CU (2006) Activated carbons and low cost adsorbents for remediation of tri- and hexavalent chromium from water. J Hazard Mater B 137:762–811
Yousefi Z, Mashayekh-Salehia A, Tahmtan RAM (2016) Biosorption of chromium in aqueous solutions using Bivalve Mollusk Shells through central composite design (CCD) model. Desalin Water Treat 57:19877–19889
Fu F, Wang Q (2011) Removal of heavy metal ions from wastewaters: a review. J Environ Manag 92:407–418
Qu X, Alvarez PJJ, Li Q (2013) Applications of nanotechnology in water and wastewater treatment. Water Res 47:3931–3946
Fang J, Gu ZM (2007) Cr(VI) removal from aqueous solution by activated carbon coated with quaternized poly(4-vinylpyridine). Environ Sci Technol 41:4748–4753
Mohan D, Singh KP, Singh VK (2006) Trivalent chromium removal from wastewater using low cost activated carbon derived from agricultural waste material and activated carbon fabric cloth. J Hazard Mater 135:280–295
US Environmental Agency, Drinking Water Contaminants. http://www.epa.gov/safewater/contaminants/index.html. 17 May 2015
Awual MR, Ismael M, Yaita T, Safty SAE, Shiwaku H, Okamoto Y, Suzuki S (2013) Trace copper(II) ions detection and removal from water using novel ligand modified composite adsorbent. Chem Eng J 222:67–76
Ahmed MT, Chaabanea T, Maachia R, Darchen A (2012) Efficiency of a pretreatment by electrocoagulation with aluminum electrodes in a nanofiltration treatment of polluted water. Procedia Eng 33:465–474
Liu C, Bai R, Ly QS (2008) Selective removal of copper and lead ions by diethylenetriamine-functionalized adsorbent: behaviors and mechanisms. Water Res 42:1511–1522
Ge F, Meng-M Li, Ye H, Bao-X Zhao (2012) Effective removal of heavy metal ions Cd2+, Zn2+, Pb2+, Cu2+ from aqueous solution by polymer-modified magnetic nanoparticles. J Hazard Mater 212:366–372
Babel S, Kurniawan TA (2003) Low-cost adsorbents for heavy metals uptake from contaminated water: a review. J Hazard Mater 45:219–243
Teh CY, Budiman PM, Shak KPY, Wu TY (2016) Recent advancement of coagulation-flocculation and its application in wastewater treatment. Ind Eng Chem Res 55:4363–4389
Bailey SE, Olin TJ, Bricka RM, Adrian DD (1999) A review of potentially low-cost sorbents for heavy metals. Water Res 33:2469–2479
Al-Rashdi B, Somerfield C, Hilal N (2011) Heavy metals removal using adsorption and nanofiltration techniques. Sep Purif Rev 40:209–259
Gatabi MP, Moghaddam HM, Ghorbani M (2016) Efficient removal of cadmium using magnetic multiwalled carbon nanotube nanoadsorbents: equilibrium, kinetic, and thermodynamic study. J Nanopart Res 18:189–206
Mobasherpour I, Salahi E, Ebrahimi M (2012) Removal of divalent nickel cations from aqueous solution by multi-walled carbon nano tubes: equilibrium and kinetic processes. Res Chem Intermed 38:2205–2222
Ihsanullah FAA, Sharkh BA, Abulkibash AM, Qureshi MI, Laoui T, Atieh MA (2016) Effect of acid modification on adsorption of hexavalent chromium (Cr(VI)) from aqueous solution by activated carbon and carbon nanotubes. Desalin Water Treat 57:7232–7244
Ihsanullah FAA, Abusharkh B, Khaled M, Atieh MA, Nasser MS, Laoui T, Agarwal S, Tyagi I, Gupta VK (2015) Adsorptive removal of cadmium(II) ions from liquid phase using acid modified carbon-based adsorbents. J Mol Liq 204:255–263
Jha VK, Matsuda M, Miyake M (2008) Sorption properties of the activated carbon-zeolite composite prepared from coal fly ash for Ni2+, Cu2+, Cd2+ and Pb2+. J Hazard Mater 160:148–153
Sekar M, Sakthi V, Rengaraj S (2004) Kinetics equilibrium adsorption study of lead (II) onto activated carbon prepared from coconut shell. J Colloid Interface Sci 279:307–313
Dahiya S, Tripathi RM, Hegde AG (2008) Biosorption of lead and copper from aqueous solutions by pre-treated crab and arca shell biomass. Bioresour Technol 99:179–187
Visa M, Duta A (2013) Methyl-orange and cadmium simultaneous removal using fly ash and photo-Fenton systems. J Hazard Mater 245:773–779
Hawari AH, Mulligan CN (2006) Biosorption of lead(II), cadmium(II), copper(II) and nickel(II) by anaerobic granular biomass. Bioresour Technol 97:692–700
Zhou Y, Nie H, Branford-White C, He Z, Zhu L (2009) Removal of Cu2+ from aqueous solution by chitosan-coated magnetic nanoparticles modified with α-ketoglutaric acid. J Colloid Interface Sci 330:29–37
Brown P, Jefcoat IA, Parrish D, Gill S, Graham S (2000) Evaluation of the adsorptive capacity of peanut hull pellets for heavy metals in solution. Adv Environ Res 4:19–29
Kadirvelu K, Brasquet CF, Cloirec PL (2000) Removal of Cu(II), Pb(II), and Ni(II) by adsorption onto activated carbon cloths. Langmuir 16:8404–8409
Reddad Z, Gerente C, Andresp Y, Ecloirec PL (2002) Adsorption of several metal ions onto a low-cost biosorbent: kinetic and equilibrium studies. Environ Sci Technol 36:2067–2073
Yavuz O, Altunkaynak Y, Güzel F (2003) Removal of copper, nickel, cobalt and manganese from aqueous solution by kaolinite. Water Res 37:948–952
Blázquez G, Hernáinz F, Calero M, Martín-Lara MA, Tenorio G (2009) The effect of pH on the biosorption of Cr(III) and Cr(VI) with olive stone. Chem Eng J 148:473–479
Rao M, Parwate AV, Bhole AG (2002) Removal of Cr6+ and Ni2+ from aqueous solution using bagasse and fly ash. Waste Manag 22:821–830
Ho YS, McKay G (1999) The sorption of lead(II) ions on peat. Water Res 33:578–584
Pan SC, Lin CC, Hwa D (2003) Reusing sewage sludge ash as adsorbent for copper removal from wastewater. Resour Conserv Recycl 39:79–90
Lu X, Wang F, Li X, Shih K, Zeng EY (2016) Adsorption and thermal stabilization of Pb2+ and Cu2+ by zeolite. Ind Eng Chem Res 55:8767–8773
Ekmekyapar F, Aslan A, Bayhan YK, Çakıcı A (2006) Biosorption of copper(II) by nonliving lichen biomass of Cladonia rangiformis hoffm. J Hazard Mater 137:293–298
Chu W (1999) Lead metal removal by recycled alum sludge. Water Res 33:3019–3025
Kim EJ, Lee CS, Chang YY, Chang YS (2013) Hierarchically structured manganese oxide-coated magnetic nanocomposites for the efficient removal of heavy metal ions from aqueous systems. ACS Appl Mater Interfaces 5:9628–9634
Akkaya T, Gülfen M, Olgun U (2013) Adsorption of rhodium(III) ions onto poly(1,8-diaminonaphthalene) chelating polymer: equilibrium, kinetic and thermodynamic study. React Funct Polym 73:1589–1596
Emik S (2014) Preparation and characterization of an IPN type chelating resin containing amino and carboxyl groups for removal of Cu(II) from aqueous solutions. React Funct Polym 75:63–74
Soykan C, Delibaş A, Coşkun R (2008) Novel copolymers of N-(4-bromophenyl)-2-methacrylamide with glycidyl methacrylate: synthesis, characterization, monomer reactivity ratios and thermal properties. React Funct Polym 68:114–124
Ali AE, El-Sawy NM, Hegazy ESA, Awadallah-F A (2011) Protein adsorption of radiation functionalized LDPE sheets. Polym Bull 67:1837–1848
Kong Z, Wei J, Li Y, Liu N, Zhang H, Zhang Y, Kong LCZ, Wei J, Lic Y, Liu N, Zhang H, Zhang Y, Cui L (2014) Rapid removal of Cr(VI) ions using quaternary ammonium fibers functioned by 2-(dimethylamino)ethyl methacrylate and modified with 1-bromoalkanes. Chem Eng J 254:365–373
Deng S, Wang P, Zhang G, Dou Y (2016) Polyacrylonitrile-based fiber modified with thiosemicarbazide by microwave irradiation and its adsorption behavior for Cd(II) and Pb(II). J Hazard Mater 307:64–72
Radovic LR, Silva IF, Ume JI, Menendez JA, Leon CA, Leon Y, Scaroni AW (1997) An experimental and theoretical study of the adsorption of aromatics possessing electron-withdrawing and electron-donating functional groups by chemically modified activated carbons. Carbon 35:1339–1348
Tang Y, Wang X, Zhu L (2013) Removal of methyl orange from aqueous solutions with poly(acrylic acid-co-acrylamide) superabsorbent resin. Polym Bull 70:905–918
Zhao Y, Yang Y, Yang S, Wang Q, Feng C, Zhang Z (2013) Adsorption of high ammonium nitrogen from wastewater using a novel ceramic adsorbent and the evaluation of the ammonium-adsorbed-ceramic as fertilizer. J Colloid Interface Sci 393:264–270
Li L, Feng X, Han R, Zang S, Yang G (2017) Cr(VI) removal via anion exchange on a silver-triazolate MOF. J Hazard Mater 321:622–628
Hazer O, Demir D (2013) Speciation of chromium in water samples by solid-phase extraction on a new synthesized adsorbent. Anal Sci 29:729–734
Abou El-Reash YG, Otto M, Kenawy İM, Ouf AM (2011) Adsorption of Cr(VI) and As(V) ions by modified magnetic chitosan chelating resin. Int J Biol Macromol 49:513–522
Panggabean AS, Yusuf B (2015) Determination of Cr(VI) by using chitosan-1,5-diphenyl carbazide resin modified at the preconcentration system with column method. Int J Pharm Bio Sci 6:101–111
Tokalıoğlu Ş, Arsav S, Delibaş A, Soykan C (2009) Indirect speciation of Cr(III) and Cr(VI) in water samples by selective separation and preconcentration on a newly synthesized chelating resin. Anal Chim Acta 645:36–41
Rengaraj S, Yeon K, Moon S (2001) Removal of chromium from water and wastewater by ion exchange resins. J Hazard Mater B 87:273–287
Hu X, Wang J, Liu Y, Li X, Zeng G, Bao Z, Zeng X, Chen A, Long F (2011) Adsorption of chromium (VI) by ethylenediamine-modified cross-linked magnetic chitosan resin: isotherms, kinetics and thermodynamics. J Hazard Mater 185:306–314
Nguyen NV, Lee J, Jeong J, Pandey BD (2013) Enhancing the adsorption of chromium(VI) from the acidic chloride media using solvent impregnated resin (SIR). Chem Eng J 219:174–182
Dalaran M, Emik S, Güçlü G, İyim TB, Özgümüş S (2009) Removal of acidic dye from aqueous solutions using poly(DMAEMA–AMPS–HEMA) terpolymer/MMT nanocomposite hydrogels. Polym Bull 63:159–171
Wen-qing W, Ming-yu L, Qing-xuan Z (2012) Thermodynamics of Cr(VI) adsorption on strong alkaline anion exchange fiber. Trans Nonferr Met Soc China 22:2831–2839
Acknowledgements
This work was supported by Research Fund of the Bozok University; Project Number 2014FBE/T113.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Coşkun, R., Er, E. & Delibaş, A. Synthesis of novel resin containing carbamothiolylimidamide group and application for Cr(VI) removal. Polym. Bull. 75, 963–983 (2018). https://doi.org/10.1007/s00289-017-2068-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-017-2068-1