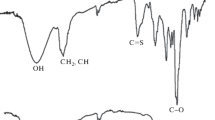
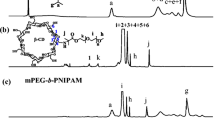
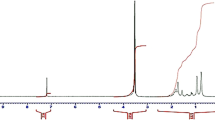
Abstract
Thermogravimetric studies of polypeptide-based hybrid copolymer (PNIPAm-g-PEG)-b-PLys in a flow of nitrogen were carried out at four rates of linear increase of the temperature. The kinetics and mechanism of the degradation process were evaluated from the TG data using the iso-conversional calculation procedure of Kissinger–Akahira–Sunose recommended by the ICTAC kinetics committee. It is very important to determine the most probable function of the mechanism, because the kinetic parameters of the process depend on its selection. In this respect, the iso-conversion calculation procedure turned out to be the most appropriate one. In the present work, the kinetic triplet (E, A and the shape of the most appropriate f(α)-function) of this process, as well as the kinetic parameters for the formation of the activated complex from the reagent, was calculated. All the calculations were performed using programs developed by us.






Similar content being viewed by others
References
Tian R, Wen X, Hai D, Yong L (2011) Sheddable micelles based on disulfide-linked hybrid PEG-polypeptide copolymer for intracellular drug delivery. Polymer 52:3580–3586. doi:10.1016/j.polymer.2011.06.013
Schmidt V, Soldi V (2006) Influence of polycaprolactone-triol addition on thermal stability of soy protein isolate based films. Polym Degrad Stab 91:3124–3130. doi:10.1016/j.polymdegradstab.2006.07.016
Tze Ch, Younsoo B, Darin F, Glen K (2012) Biodegradable hybrid recombinant block copolymers for non-viral gene transfection. Int J Pharm 427:105–112. doi:10.1016/j.ijpharm.2011.09.035
Georgieva V, Zvezdova D, Vlaev L (2013) Non-isothermal kinetics of thermal degradation of chitin. J Therm Anal Calorim 111:763–771. doi:10.1007/s10973-012-2359-6
Bigda R, Mianowski A (2006) Influence of heating rate on kinetic quantities of solid phase thermal decomposition. J Therm Anal Calorim 84(2):453–465. doi:10.1007/s10973-005-7378-0
Yeganeh H, Lakouraj M, Jamshidi S (2005) Synthesis and properties of biodegradable elastomeric epoxy modified polyurethanes based on poly(e-caprolactone) and poly(ethylene glycol). Eur Polym J 41:2370–2379. doi:10.1016/j.eurpolymj.2005.05.004
Fr Signori, Chiellini F, Solaro R (2005) New self-assembling biocompatible–biodegradable amphiphilic block copolymers. Polymer 46:9642–9652. doi:10.1016/j.polymer.2005.07.071
Tsai MC, Shih CM, Lue SJ (2013) Drug permeation behavior through thermo- and pH-responsive polycarbonate-g-poly(N-isopropylacrylamide-co-acrylic acid) composites. Polym Bull 70:1003–1017. doi:10.1007/s00289-012-0865-0
Bikiaris D (2011) Can nanoparticles really enhance thermal stability of polymers Part II: An overview on thermal decomposition of polycondensation polymers. Thermochim Acta 523:25–45. doi:10.1016/j.tca.2011.06.012
Czech Zb, Kowalczyk A, Kabatc J, Swiderska J (2013) Thermal stability of poly(2-ethylhexyl acrylates) used as plasticizers for medical application. Polym Bull 70:1911–1918. doi:10.1007/s00289-012-0887-7
Cekingen S, Saltan F, Yildirim Y, Akat H (2012) A novel HEMA-derived monomer and copolymers containing side-chain thiophene units: synthesis, characterization and thermal degradation kinetics. Thermochim Acta 546:87–93. doi:10.1016/j.tca.2012.07.027
Turmanova S, Genieva S, Dimitrova A, Vlaev L (2008) Non-isothermal degradation kinetics of filled with rice husk ash polypropene composites. eXPRESS Polym Lett 2(2):133–146. doi:10.3144/expresspolymlett.2008.18
Vlaev L, Turmanova S, Genieva S (2009) Chapter 12. In: Donahue WS, Brandt JC (eds) Products and applications of pyrolyzed rice husks: structure, morphology, thermal, kinetics and physicomechanical characteristics, in pyrolysis: types, processes, and industrial sources and products. Nova Science Publishers, New York, pp 267–323
Genieva S, Turmanova S, Vlaev L (2011) Chapter 13. In: Kalia K, Kaith G, Kaur S (eds) Utilization of rice husks and the products of its degradation as fillers of polymers in cellulose fibers, bio-, and nano-polymer composites. Part II: cellulosic fiber reinforced polymer composites. Springer AG, Germany, pp 345–376
Ivanova E, Dimitrov I, Kozarova R, Turmanova S, Apostolova M (2013) Thermally sensitive polypeptide-based copolymer for DNA complexation into stable nanosized polyplexes. J Nanopart Res 15(1):1358. doi:10.1007/s11051-012-1358-7
Atanassov A, Genieva S, Vlaev L (2010) Study of the thermooxidative degradation kinetics of tetrafluoroethylene-ethylene copolymer filled with rice husks ash. Polym-Plast Technol Eng 49:541–554. doi:10.1080/03602550903532224
Vlaev L, Nedelchev N, Gyurova K, Zagorcheva M (2008) A comparative study of non-isothermal kinetics of decomposition of calcium oxalate monohydrate. J Anal Appl Pyrol 81(2):253–262. doi:10.1016/j.jaap.2007.12.003
Coats AW, Redfern JP (1964) Kinetic parameters from thermogravimetric data. Nat Lond 201:68–69. doi:10.1038/201068a0
Tang W, Liu Y, Zhang H, Wang C (2003) New approximate formula for Arrhenius temperature integral. Thermochim Acta 408:39–43. doi:10.1016/S0040-6031(03)00310-1
Wanjun T, Cunxin W, Donghua C (2005) Kinetic studies on the pyrolysis of chitin and chitosan. Polymer Degrad Stabil 87:389–394. doi:10.1016/j.polymdegradstab.2004.08.006
Boonchom B, Thongkam M (2010) Kinetics and thermodynamics of the formation of MnFeP4O12. J Chem Eng Data 55:211–216. doi:10.1021/je900310m
Vyazovkin S (2006) Model-free kinetics. Staying free of multiplying entities without necessity. J Therm Anal Calorim 83(1):45–51. doi:10.1007/s10973-005-7044-6
Genieva S, Vlaev L, Atanassov A (2010) Study of the thermooxidative degradation kinetics of poly(tetrafluoroethene) using iso-conversional calculation procedure. J Therm Anal Calorim 99(2):551–561. doi:10.1007/s10973-009-0191-4
Su T-T, Jiang H, Gong H (2008) Thermal stabilities and the thermal degradation kinetics of poly(ε-caprolactone). Polym Plast Technol Eng 47:398–403. doi:10.1080/03602550801897695
Vyazovkin S, Burnham A, Criado J, Perez-Maqueda L, Popescu C, Sbirrazzuoli N (2011) ICTAC kinetics committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta 520:1–19. doi:10.1016/j.tca.2011.03.034
Marian E, Tiţa B, Jurca T, Fulias A, Vicas L, Tiţa D (2012) Thermal behaviour of erythromycin-active substance and tablets Part 1: Kinetic study of the active substance under non-isothermal conditions. J Thermal Anal Calorim. doi:10.1007/s10973-012-2284-8
He W, Deng F, Liao G-X et al (2010) Kinetics of thermal degradation of poly(aryl ether) containing phthalazinone and life estimation. J Therm Anal Calorim 100:1055–1062. doi:10.1007/s10973-009-0515-4
Kissinger HE (1957) Reaction kinetics in different thermal analysis. J Anal Chem 29:1702–1706
Senum G, Yang R (1977) Rational approximations of the integral of the Arrhenius function. J Therm Anal Calorim 11:445–447. doi:10.1007/BF01903696
Albano CL, Sciamanna ES, AqunoT, Martinez JJ (2000) Methodology to evaluate thermogravimetric data using computational techniques in the polymer field. In: European congress on computational methods in applied science and engineering, ECCOMAS 2000, Barcelona, Spain
Georgieva V, Zvezdova D, Vlaev L (2012) Non-isothermal kinetics of thermal degradation of chitosan. 6: art. Chem Cent J 1(81):1–10. doi:10.1186/1752-153X-6-81
Georgieva V, Vlaev L, Gyurova K (2013) Non-isothermal degradation kinetics of CaCO3 from different origin. J Chem Art 872981:1–12
Corders H (1968) The preexponential factors for solid-state thermal decomposition. J Phys Chem 72(6):2185–2189
Singh LK, Mitra S (1989) Thermal investigation and stereochemical studies of some cyclic diamine complexes of nickel(II), zinc(II) and cadmium(II) in the solid state. part II. Thermochim Acta 138:285–301. doi:10.1016/0040-6031(89)87265-X
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ivanova, E.D., Georgieva, V.G., Chr. Turmanova, S. et al. Characterization of hybrid copolymer containing thermosensitive and polypeptide blocks by thermogravimetric analysis. Polym. Bull. 71, 167–179 (2014). https://doi.org/10.1007/s00289-013-1052-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-013-1052-7