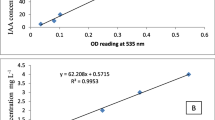
Abstract
This work aimed to assess the ability of plant growth-promoting Bacilli isolated from wheat rhizosphere and rock phosphate mine soils to convert inorganic phosphate (Pi) from Moroccan natural phosphate (NP) to soluble forms. The effect of these bacteria on wheat plants in order to increase their phosphorus (P) uptake in vitro was also investigated. Bacteria were isolated from wheat rhizosphere and natural rock phosphate soils and screened for their ability to solubilize Tri-Calcium Phosphate (TCP) and Natural Rock Phosphate (NP), to produce indole-3-acetic acid (IAA), siderophores and 1-aminocyclopropane-1-carboxylate (ACC) deaminase. Isolates were identified by 16S rRNA sequencing and tested for their capacity to increase wheat plants growth and their phosphorus uptake.Twenty-four strains belonging to Bacillus genus isolated from both biotopes were screened for their ability to solubilize Pi. The highest NP solubilization was showed by strains isolated from wheat rhizosphere. Solubilization of Pi was accompanied by organic acid production. Strains produce IAA, siderophore and ACC deaminase. Inoculation assays using efficient NP-solubilizing bacilli strains from both sources showed the ability of these isolates to increase wheat growth and the phosphorus uptake under in vitro conditions. Bacilli strains isolated from rhizosphere soil and natural rock phosphorus soil showed effective solubilization of Pi from rock phosphate. Phosphate solubilizing Bacilli were evaluated for their plant growth promotion under in vitro conditions. Results revealed the positive effect of all strains on biometric parameters and P content of wheat seedlings.





Similar content being viewed by others
Abbreviations
- TCP:
-
Tri-calcium phosphate
- NP:
-
Natural rock phosphate
- IAA:
-
Indole-3-acetic acid
- Pi:
-
Inorganic phosphate
- ACC deaminase:
-
1-Aminocyclopropane-1-carboxylate (ACC) deaminase
- RPO:
-
Rock Phosphate Ore
- UPLC:
-
Ultra performance liquid chromatography
- PSM:
-
Phosphate solubilizing microorganisms
- PGPR:
-
Plant growth promotion-rhizobacteria
- LB:
-
Lysogeny brothmedium
- TSB:
-
Tryptone soy broth
- CMC:
-
Carboxymethyl cellulose
- MS:
-
Murashige and skoog
References
FAO (2015) The future of food and agriculture: trends and challenges
Bregaglio S, Frasso N, Pagani V et al (2015) New multi-model approach gives good estimations of wheat yield under semi-arid climate in Morocco. Agron Sustain Dev 35:157–167. https://doi.org/10.1007/s13593-014-0225-6
Sharma SB, Sayyed RZ, Trivedi MH, Gobi TA (2013) Phosphate solubilizing microbes: sustainable approach for managing phosphorus deficiency in agricultural soils. SpringerPlus 2:587. https://doi.org/10.1186/2193-1801-2-587
Vassilev N, Vassileva M, Nikolaeva I (2006) Simultaneous P-solubilizing and biocontrol activity of microorganisms: potentials and future trends. Appl Microbiol Biotechnol 71:137–144. https://doi.org/10.1007/s00253-006-0380-z
Wakelin SA, Warren RA, Harvey PR, Ryder MH (2004) Phosphate solubilization by Penicillium spp. closely associated with wheat roots. Biol Fertil Soils 40:36–43. https://doi.org/10.1007/s00374-004-0750-6
Rajan SSS, Watkinson JH, Sinclair AG (1996) Phosphate rocks for direct application to soils. In: Sparks DL (ed) Advances in agronomy. Academic Press, Cambridge, pp 77–159
Egamberdieva D, Kamilova F, Validov S, Gafurova L, Kucharova Z, Lugtenberg BL (2008) High incidence of plant growth-stimulating bacteria associated with the rhizosphere of wheat grown on salinated soil in Uzbekistan. Environ Microbiol 10:1–9. https://doi.org/10.1111/j.1462-2920.2007.01424.x
Bhattacharyya PN, Jha DK (2012) Plant growth-promoting rhizobacteria ( PGPR ): emergence in agriculture. World J Microbiol Biotechnol. https://doi.org/10.1007/s11274-011-0979-9
Meftah I, Lamiaa K, Salah C et al (2018) Phosphate-solubilizing and auxin-producing Rhizobacteria promote plant growth under saline conditions. Arab J Sci Eng. https://doi.org/10.1007/s13369-017-3042-9
Gauri SS, Mandal SM, Mondal KC, Dey S, Pati BR (2009) Enhanced production and partial characterization of an extracellular polysaccharide from newly isolated Azotobacter sp. SSB81. Bioresour Technol 100:4240–4243. https://doi.org/10.1016/j.biortech.2009.03.064
Chun J, Lee J, Jung Y, Kim M, Kim S, Kim BK, Lim Y (2007) EzTaxon : a web-based tool for the identification of prokaryotes based on 16S ribosomal RNA gene sequences. Int J Syst Evol Microbiol 57(10):2259–2261. https://doi.org/10.1099/ijs.0.64915-0
Loper J (1986) Influence of bacterial sources of indole3-acetic acid on root elongation of sugar beet. Phytopathology. https://doi.org/10.1094/Phyto-76-386
Penrose DM, Glick BR (2003) Methods for isolating and characterizing ACC deaminase-containing plant growth-promoting rhizobacteria. Physiol Plant 118:10–15. https://doi.org/10.1034/j.1399-3054.2003.00086.x
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Schwyn B, Neilands JB (1987) Universal chemical assay for the detection and determination of siderophores. Anal Biochem 160:47–56. https://doi.org/10.1016/0003-2697(87)90612-9
Murashige T, Skoog F (1962) A Revised medium for eapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x11
Sonenshein AL, Hoch JA, Losick R (1993) Bacillus subtilis and other gram-positive bacteria: from genes to cells. Am Soc Microbiol, p 1020. ISBN :1555810535
Bouizgarne B. (2013). Bacteria for plant growth promotion and disease management. In Maheshwari DK (ed) Bacteria in agrobiology: disease management, pp 15–47 (33p). ISBN: 978-3-642-33638-6; DOI 10.1007/978-3-642-33639-3_2
Pérez-García A, Romero D, de Vicente A (2011) Plant protection and growth stimulation by microorganisms: biotechnological applications of Bacilli in agriculture. Curr Opin Biotechnol 22:187–193. https://doi.org/10.1016/j.copbio.2010.12.003
Tofazal MI, Rahman M, Pandey P, Jha CK, Aeron A (2017) Bacilli and Agrobiotechnology. Springer. ISBN 978–3–319–44409–3
Kumar P, Dubey RC, Maheshwari DK (2012) Bacillus strains isolated from rhizosphere showed plant growth promoting and antagonistic activity against phytopathogens. Microbiol Res 167:493–499. https://doi.org/10.1016/j.micres.2012.05.002
Han HS, Supanjani LKD (2006) Effect of co-inoculation with phosphate and potassium solubilizing bacteria on mineral uptake and growth of pepper and cucumber. Plant Soil Environ 52:130–136. https://doi.org/10.17221/3356-PSE
Vivas A, Barea JM, Azcón R (2005) Brevibacillus brevis isolated from Cadmium- or Zinc-contaminated soils Improves in Vitro spore germination and growth of Glomus mosseae under High Cd or Zn concentrations. Microb Ecol 49:416–424. https://doi.org/10.1007/s00248-004-0044-4
Chen YP, Rekha PD, Arun AB, Shen FT, Lai WA, Young CC (2006) Phosphate solubilizing bacteria from subtropical soil and their tricalcium phosphate solubilizing abilities. Appl Soil Ecol 34:33–41. https://doi.org/10.1016/j.apsoil.2005.12.002
Swain MR, Laxminarayana K, Ray RC (2012) Phosphorus solubilization by thermotolerant Bacillus subtilis isolated from cow dung microflora. Agric Res 1:273–279. https://doi.org/10.1007/s40003-012-0022-x
Gupta R, Singal R, Shankar A, Kuhad RC, Saxena RK (1994) A modified plate assay for screening phosphate solubilizing microorganisms. J Gen Appl Microbiol 40:255–260. https://doi.org/10.2323/jgam.40.255
Bashan Y, Kamnev AA, de Bashan LE (2013) Tricalcium phosphate is inappropriate as a universal selection factor for isolating and testing phosphate-solubilizing bacteria that enhance plant growth: a proposal for an alternative procedure. Biol Fertil Soils 49:465–479. https://doi.org/10.1007/s00374-012-0737-7
Chang C, Yang S (2009) Bioresource technology thermo-tolerant phosphate-solubilizing microbes for multi-functional biofertilizer preparation. Bioresour Technol 100:1648–1658. https://doi.org/10.1016/j.biortech.2008.09.009
Kumari AK, Kapoor KS, Kundu B, Kumari Mehta R (2008) Identification of organic acids produced during rice straw decomposition and their role in rock phosphate solubilization. Plant Soil Environ. https://doi.org/10.17221/2783-PSE
Yadav H, Gothwal RK, Nigam VK, Sinha-Roy S, Ghosh P (2013) Optimization of culture conditions for phosphate solubilization by a thermo-tolerant phosphate-solubilizing bacterium Brevibacillus sp. BISR-HY65 isolated from phosphate mines. Biocatal Agric Biotechnol 2:217–225. https://doi.org/10.1016/j.bcab.2013.04.005
Saeid A, Prochownik E, Dobrowolska-Iwanek J (2018) Phosphorus solubilization by Bacillus species. Molecules 23:2897. https://doi.org/10.3390/molecules23112897
Reyes I, Valery A, Valduz Z (2006) Phosphate-solubilizing microorganisms isolated from rhizospheric and bulk soils of colonizer plants at an abandoned rock phosphate mine. Plant Soil 287:69–75. https://doi.org/10.1007/s11104-006-9061-z
Rodríguez H, Fraga R (1999) Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol Adv 17:319–339. https://doi.org/10.1016/S0734-9750(99)00014-2
Vyas P, Gulati A (2009) Organic acid production in vitro and plant growth promotion in maize under controlled environment by phosphate-solubilizing fluorescent Pseudomonas. BMC Microbiol 9:174. https://doi.org/10.1186/1471-2180-9-174
Ben Farhat M, Farhat A, Bejar W et al (2009) Characterization of the mineral phosphate solubilizing activity of Serratia marcescens CTM 50650 isolated from the phosphate mine of Gafsa. Arch Microbiol 191:815–824. https://doi.org/10.1007/s00203-009-0513-8
Duca D, Lorv J, Patten CL, Rose D, Glick BR (2014) Indole-3-acetic acid in plant–microbe interactions. Antonie Van Leeuwenhoek 106:85–125. https://doi.org/10.1007/s10482-013-0095-y
Kumar A, Kumar A, Pratush A (2014) Molecular diversity and functional variability of environmental isolates of Bacillus species. SpringerPlus 3:312. https://doi.org/10.1186/2193-1801-3-312
Wahyudi A, Puji Astuti R, Widyawati A et al (2011) Characterization of Bacillus sp. strains isolated from rhizosphere of soybean plants for their use as potential plant growth for promoting Rhizobacteria. J Microbiol Antimicrob 3:34–40
Sachdev D, Chaudhari H, Kasture MV et al (2009) Isolation and characterization of indole acetic acid (IAA) producing Klebsiella pneumonia strains from rhizosphere of wheat (Triticum aestivum) and their effect on plant growth. Indian J Exp Biol 47:993–1000
Arruda L, Beneduzi A, Martins A, Lisboa B, Lopes C, Bertolo F, Vargas LK (2013) Screening of rhizobacteria isolated from maize ( Zea mays L.) in Rio Grande do Sul State (South Brazil) and analysis of their potential to improve plant growth. Appl Soil Ecol 63:15–22. https://doi.org/10.1016/j.apsoil.2012.09.001
Van de Poel B, Van Der Straeten D (2014) 1-aminocyclopropane-1-carboxylic acid ( ACC ) in plants : more than just the precursor of ethylene ! Front Plant Sci 5:640. https://doi.org/10.3389/fpls.2014.00640
Baig KS, Arshad M, Shaharoona B et al (2012) Comparative effectiveness of Bacillus spp. possessing either dual or single growth-promoting traits for improving phosphorus uptake, growth and yield of wheat (Triticum aestivum L.). Ann Microbiol 62:1109–1119. https://doi.org/10.1007/s13213-011-0352-0
Lim J-H, An C-H, Kim Y-H et al (2012) Isolation of auxin- and 1-aminocyclopropane-1-carboxylic acid deaminase-producing bacterium and its effect on pepper growth under saline stress. J Korean Soc Appl Biol Chem 55:607–612. https://doi.org/10.1007/s13765-012-2097-2
Nain L, Yadav RC, Saxena J (2012) Characterization of multifaceted Bacillus sp. RM-2 for its use as plant growth promoting bioinoculant for crops grown in semi arid deserts. Appl Soil Ecol 59:124–135. https://doi.org/10.1016/j.apsoil.2011.08.001
Singh RP, Jha PN (2016) Ahalotolerant bacterium Bacillus licheniformis HSW-16 augments induced systemic tolerance to salt stress in wheat plant (Triticum aestivum) isolation of bacteria. Front Plant Sci 7:1–18. https://doi.org/10.3389/fpls.2016.0189046
Noreen S, Ali B, Hasnain S (2012) Growth promotion of Vigna mungo (L.) by Pseudomonas spp. exhibiting auxin production and ACC-deaminase activity. Ann Microbiol 62:411–417. https://doi.org/10.1007/s13213-011-0277-7
Sarker A, Talukder NM, Islam MT (2014) Phosphate solubilizing bacteria promote growth and enhance nutrient uptake by wheat. Plant Sci Today 1(2):86–93. https://doi.org/10.14719/pst.2014.1.2.25
Swarnalakshmi K, Prasanna R, Kumar A, Pattnaik S (2013) Evaluating the in fl uence of novel cyanobacterial bio fi lmed biofertilizers on soil fertility and plant nutrition in wheat. Eur J Soil Biol 55:107–116. https://doi.org/10.1016/j.ejsobi.2012.12.008
Hamdali H, Hafidi M, Virolle MJ, Ouhdouch Y (2008) Growth promotion and protection against damping-off of wheat by two rock phosphate solubilizing actinomycetes in a P-deficient soil under greenhouse conditions. Appl Soil Ecol 40:510–517. https://doi.org/10.1016/j.apsoil.2008.08.001
Acknowledgements
The Authors would like to acknowledge the support through the R&D Initiative – Appel à projets autour des phosphates APPHOS – sponsored by OCP (OCP Foundation, R&D OCP, Mohammed VI Polytechnic University, National Center of Scientific and technical Research CNRST, Ministry of Higher Education, Scientific Research and Professional Training of Morocco MESRSFC) under the project entitled * Bioformulation de Consortium de Microorganismes solubilisateurs du phosphate: effets bénéfiques sur la croissance et la protection des plantes *, project ID * BIO-BIZ-01/2017*”
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Azaroual, S.E., Hazzoumi, Z., Mernissi, N.E. et al. Role of Inorganic Phosphate Solubilizing Bacilli Isolated from Moroccan Phosphate Rock Mine and Rhizosphere Soils in Wheat (Triticum aestivum L) Phosphorus Uptake. Curr Microbiol 77, 2391–2404 (2020). https://doi.org/10.1007/s00284-020-02046-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-020-02046-8