Abstract
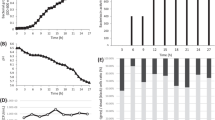
This work focuses on the biological understanding of the biocontrol agent Bacillus amyloliquefaciens CPA-8 in order to accomplish the characterization required in the registration process for the development of a microorganism-based product. The tolerance of CPA-8 to grow under different pH–temperature and water activity (a w)–temperature conditions was widely demonstrated. Regarding the pH results, optimum growth at the evaluated conditions was observed at 37 °C and pH between 7 and 5. On the contrary, the slowest growth was recorded at 20 °C and pH 4.5. Moreover, the type of solute used to reduce a w had a great influence on the minimum a w at which the bacterium was able to grow. The lowest a w values for CPA-8 growth in media modified with glycerol and glucose were 0.950 and 0.960, respectively. Besides, the lowest a w for CPA-8 growth increased when the temperature decreased to 20 °C, at which CPA-8 was not able to grow at less than 0.990 a w, regardless of the type of solute. Antibiotic susceptibility tests were carried out to determine which antibiotic could affect the behavior of the bacteria and revealed that CPA-8 was clearly resistant to hygromycin. Finally, a PCR amplification assay to detect the presence of enterotoxic genes from Bacillus cereus in CPA-8 was also performed. CPA-8 gave negative results for all the genes tested except for nheA gene, which is not enough for the toxicity expression, suggesting that fruit treated with this antagonist will not be a potential vehicle for foodborne illnesses.




Similar content being viewed by others
References
Baranyi J, Roberts TA (1994) A dynamic approach to predicting bacterial growth in food. Int J Food Microbiol 23(3):277–294
Beattie SH, Williams AG (1999) Detection of toxigenic strains of Bacillus cereus and other Bacillus spp. with an improved cytotoxicity assay. Lett Appl Microbiol 28(3):221–225
Casals C, Teixidó N, Viñas I, Silvera E, Lamarca N, Usall J (2010) Combination of hot water, Bacillus subtilis CPA-8 and sodium bicarbonate treatments to control postharvest brown rot on peaches and nectarines. Eur J Plant Pathol 128(1):51–63
Costa E, Usall J, Teixidó N, Delgado J, Viñas I (2002) Water activity, temperature, and pH effects on growth of the biocontrol agent Pantoea agglomerans CPA-2. Can J Microbiol 48:1082–1088
Crespo-Sempere A, Estiarte N, Marín S, Sanchis V, Ramos AJ (2013) Propidium monoazide combined with real-time quantitative PCR to quantify viable Alternaria spp. contamination in tomato products. Int J Food Microbiol 165(3):214–220
Dallyn H, Fox A (1980) Spoilage of material of reduced water activity by xerophilic fungi. In: Gould GH, Corry JEL (eds) Society of applied bacteriology technical series. Academic Press Ltd., London, pp 129–139
Droby S, Wisniewski M, Teixidó N, Spadaro D, Jijakli MH (2016) The science, development, and commercialization of postharvest biocontrol products. Postharvest Biol Technol 122:22–29
Garrett KA, Dendy SP, Frank EE, Rouse MN, Travers SE (2006) Climate change effects on plant disease: genomes to ecosystems. Annu Rev Phytophathol 44:489–509
Gotor-Vila A, Teixidó N, Di Francesco A, Usall J, Ugolini L, Torres R, Mari M (2017) Antifungal effect of volatile organic compounds produced by Bacillus amyloliquefaciens CPA-8 against fruit pathogen decays of cherry. Food Microbiol 64:219–225
Gotor-Vila A, Teixidó N, Usall J, Dashevskaya S, Torres R (2016) Development of a SCAR marker and a strain-specific genomic marker for the detection of the biocontrol agent strain CPA-8 Bacillus amyloliquefaciens (formerly B. subtilis). Ann Appl Biol 169:248–256
Hansen BM, Hendriksen NB (2001) Detection of enterotoxic Bacillus cereus and Bacillus thuringiensis strains by PCR analysis. Appl Environ Microbiol 67(1):185–189
Kadaikunnan S, Rejiniemon TS, Khaled JM, Alharbi NS, Mothana R (2015) In-vitro antibacterial, antifungal, antioxidant and functional properties of Bacillus amyloliquefaciens. Ann Clin Microbiol Antimicrob 14:9
Kumar TDK, Murali HS, Batra HV (2010) Multiplex PCR assay for the detection of enterotoxic Bacillus cereus group strains and its application in food matrices. Indian J Microbiol 50(2):165–171
Lindbäck T, Fagerlund A, Rødland MS, Granum PE (2004) Characterization of the Bacillus cereus Nhe enterotoxin. Microbiology 150:3959–3967
López AC, Alippi AM (2010) Enterotoxigenic gene profiles of Bacillus cereus and Bacillus megaterium isolates recovered from honey. Rev Argent Microbiol 42:216–225
Loshon CA, Wahome PG, Maciejewski MW, Setlow P (2006) Levels of glycine betaine in growing cells and spores of Bacillus species and lack of effect of glycine betaine on dormant spore resistance. J Bacteriol 188(8):3153–3158
Mari M, Torres R, Casalini L, Lamarca N, Mandrin JF, Lichou J, Larena I, De Cal MA, Melgarejo P, Usall J (2007) Control of post-harvest brown rot on nectarine by Epicoccum nigrum and physico-chemical treatments. J Sci Food Agric 87(7):1271–1277
Medina A, Magan N (2010) Comparisons of water activity and temperature impacts on growth of Fusarium langsethiae strains from northern Europe on oat-based media. Int J Food Microbiol 142(3):365–369
Mossel DAA, Corry JEL, Struijk CB, Baird RM (1995) Essentials of the microbiology of foods: a textbook for advanced studies. Wiley, New York
Ngamwongsatit P, Buasri W, Pianariyanon P, Pulsrikam C, Ohba M, Assavanig A, Panbangred W (2008) Broad distribution of enterotoxin genes (hblCDA, nheABC, cytK, and entFM) among Bacillus thuringiensis and Bacillus cereus as shown by novel primers. Int J Food Microbiol 121(3):352–356
Nunes C (2012) Biological control of postharvest diseases of fruit. Eur J Plant Pathol 133(1):181–196
Padan E, Bibi E, Ito M, Krulwich TA (2005) Alkaline pH homeostasis in bacteria: new insights. Biochimica Et Biophysica Acta-Biomembranes 1717(2):67–88
Phelps RJ, McKillip JL (2002) Enterotoxin production in natural isolates of Bacillaceae outside the Bacillus cereus group. Appl Environ Microbiol 68(6):3147–3151
Ratkowsky DA, Olley J, McMeekin TA, Ball A (1982) Relationship between temperature and growth rate of bacterial cultures. J Bacteriol 149(1):1–5
Scott WJ (1957) Water relations of food spoilage microorganisms. Adv Food Res 7:83–127
Sharma RR, Singh D, Singh R (2009) Biological control of postharvest diseases of fruits and vegetables by microbial antagonists: a review. Biol Control 50(3):205–221
Sisquella M, Casals C, Vinas I, Teixido N, Usall J (2013) Combination of peracetic acid and hot water treatment to control postharvest brown rot on peaches and nectarines. Postharvest Biol Technol 83:1–8
Teixidó N, Cañamás TP, Usall J, Torres R, Magan N, Viñas I (2005) Accumulation of the compatible solutes, glycine–betaine and ectoine, in osmotic stress adaptation and heat shock cross-protection in the biocontrol agent Pantoea agglomerans CPA-2. Lett Appl Microbiol 41(3):248–252
Teixidó N, Torres R, Abadias M, Usall J (2011) Biological control of postharvest diseases in fruit and vegetables. In: Lacroix C (ed) Protective cultures, antimicrobial metabolites and bacteriophages for food and beverage. Woodhead Publishing Limited, Cambridge, pp 364–402
Teixidó N, Viñas I, Usall J, Sanchis V, Magan N (1998) Ecophysiological responses of the biocontrol yeast Candida sake to water, temperature and pH stress. J Appl Microbiol 84(2):192–200
Usall J, Casals C, Sisquella M, Palou L, De Cal A (2015) Alternative technologies to control postharvest diseases of stone fruits. Stewart Postharvest Rev 11(4):1–6
Yánez-Mendizábal V, Usall J, Viñas I, Casals C, Marín S, Solsona C, Teixidó N (2011) Potential of a new strain of Bacillus subtilis CPA-8 to control the major postharvest diseases of fruit. Biocontrol Sci Technol 21(4):409–426
Yánez-Mendizábal V, Viñas I, Usall J, Torres R, Solsona C, Teixidó N (2012) Production of the postharvest biocontrol agent Bacillus subtilis CPA-8 using low cost commercial products and by-products. Biol Control 60(3):280–289
Yánez-Mendizábal V, Zeriouh H, Viñas I, Torres R, Usall J, de Vicente A, Pérez-García A, Teixidó N (2012) Biological control of peach brown rot (Monilinia spp.) by Bacillus subtilis CPA-8 is based on production of fengycin-like lipopeptides. Eur J Plant Pathol 132(4):609–619
Acknowledgments
This research was supported by the European project BIOCOMES FP7-612713 and by the Secretaria d’Universitats i Recerca del Departament d’Economia i Coneixement de la Generalitat de Catalunya for the PhD Grant 2014-FI-B00367 (Amparo M. Gotor Vila). The authors also thank CERCA Program (Generalitat de Catalunya).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interest.
Rights and permissions
About this article
Cite this article
Gotor-Vila, A., Teixidó, N., Sisquella, M. et al. Biological Characterization of the Biocontrol Agent Bacillus amyloliquefaciens CPA-8: The Effect of Temperature, pH and Water Activity on Growth, Susceptibility to Antibiotics and Detection of Enterotoxic Genes. Curr Microbiol 74, 1089–1099 (2017). https://doi.org/10.1007/s00284-017-1289-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-017-1289-8